Synthesis and reaction behavior of calcium silicate hydrate in basic system
LIU Gui-hua(刘桂华), HE Qiang(贺 强), LI Xiao-bin(李小斌),
PENG Zhi-hong(彭志宏), ZHOU Qiu-sheng(周秋生)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: At the molar ratio of CaO to SiO2 of 1, with calcium hydroxide and sodium silicate, calcium silicate hydrate was synthesized at 50, 100, 170℃, respectively. The results show that temperature favors the formation of calcium silicate hydrate with perfect structure. When calcium silicate hydrate reacts with caustic solution, the decomposition rate of calcium silicate hydrate increases with the increasing caustic concentration and decreases with the raising synthesis temperature and the prolongation of reaction time. The decomposition rate is all less than 1.2% in caustic solution, and XRD pattern of the residue after reaction with caustic solution is found as the same as that of original calcium silicate hydrate, which indicates the stable existence of calcium silicate hydrate in caustic solution. When reacted with soda solution, the decomposition rate increases with the increasing soda concentration and reaction time, while decreases with the synthesis temperature. The decomposition rate is more than 2% because CaO·SiO2·H2O(CSH(Ⅰ)), except Ca5(OH)2Si6O16·4H2O and Ca6Si6O17(OH)2, is decomposed. So the synthesis temperature and soda concentration should be controlled in the process of transformation of sodium aluminosilicate hydrate into calcium silicate hydrate.
Key words: calcium silicate hydrate; reaction behavior; soda solution CLC number: TF821
Document code: A
1 INTRODUCTION
Its key to separate alumina from silica in alumina production by Bayer or sintering process. Whether silica changes to sodium aluminosilicate hydrate or hydro-garnet(both titled desilication product, DSP), both contain soda or alumina. If DSP is discharged out directly, a large amount of soda and alumina will be wasted, meanwhile discharged DSP will result in heavy environmental pollution. In present it costs a lot to recover alumina and soda from DSP by sintering process[1]. So it is urgent to develop new hydro-process technology to recover alumina and soda by formation of calcium silicate hydrate(mCaO·nSiO2·xH2O), and then the technology of sinter process can be simplified, alumina can be produced with bauxite containing high silica by Bayer process as well.
Though there exist various kinds of calcium silicate hydrate, the formation and reaction behaviors havent been studied systemically in concentrated caustic solution yet. In the past, much research on calcium silicate hydrate was focused on the hydro-chemical technology at high pressure, and calcium silicate hydrate occurred at high temperature(>250℃), high molar ratio of caustic to alumina and low caustic concentration[2, 3]. Various kinds of calcium silicate hydrate were reported in cement industry, but the condition of its formation is fairly different from that of alumina production. For instance, the temperature, in general, is less than 50℃ and caustic concentration is very low in cement industry[4-10].
In this paper three kinds of calcium silicate hydrate are synthesized, and their reaction behaviors between calcium silicate hydrate and soda(or caustic) solution are investigated in detail.
2 EXPERIMENTAL
An aqueous solution with the concentration of 7g/L SiO2 and around 50g/L Na2O was prepared by dissolving sodium silicate(analytical pure) and sodium hydroxide(analytical pure) into distilled water. According to a molar ratio CaO to SiO2 of 1, ground calcium hydroxide(analytical pure) was added into the above mentioned sodium silicate solution. After reaction 5h, the slurry was filtrated, the slag was washed with boiling water, and dried at about 100℃, and then the calcium silicate hydrate was obtained.
A specific amount of calcium silicate hydrate was weighed according to the value of mass ratio of liquid to solid, and then added into caustic or soda solution which was placed in a reactor(glycerin is used as heat transfer media, temperature range is controlled within 1℃). After a period of reaction, slurry was filtrated, concentration of SiO2 was measured in filtrate, the decomposition rate of calcium silicate hydrate was also calculated (the mass ratio of SiO2 in solution to original SiO2 in calcium silicate hydrate, expressed by η(SiO2)). At the same time, the filter cake was washed and dried to be analyzed.
The concentration of SiO2 in solution was analyzed by D7230G spectrophotometer(Shanghai, China), concentration of Na2O in solution was analyzed by titration, and concentration of Na2O in solid was analyzed by FP640 flame photometer(Shanghai, China).The XRD was made by D500(Siemens) scanning consecutively at a speed of 40°/min, and 2θ varied from 10° to 80°.
3 RESULTS AND DISCUSSION
3.1 Synthesis of calcium silicate hydrate
Synthesis condition and composition of calcium silicate hydrate were shown in Table 1.
The results indicate that the molar ratio of CaO to SiO2 of calcium silicate hydrate at normal pressure were about 1, but 1.27 at high pressure, this might be due to the transformation of tobermorite(Ca5Si6O16(OH)2·4H2O) into gyrolite(Ca8(Si4O10)3(OH)4·6H2O) or xonotlite(Ca6Si6O17(OH)2); etc[11, 12]. So temperature affects the composition and structure of calcium silicate hydrate.
Meanwhile, though the caustic concentration was about 50g/L, the content of Na2O in calcium silicate hydrate were less than 2.2%. It meant little pectolite was formed[9, 11, 13].
3.2 Reaction behavior of calcium silicate hydrate in caustic or soda solution
3.2.1 Reaction of calcium silicate hydrate in caustic solution
Reaction equation of calcium silicate hydrate in caustic solution can be expressed as
CaO·SiO2·nH2O+2OH-= Ca(OH)2+SiO2(OH)2-2+(n-1)H2O
Stability of calcium silicate hydrate in caustic concentration is the key factor of affecting solubility of calcium silicate hydrate. The reaction behavior is shown in Fig.1.
The results in Fig.1 show that whether calcium silicate hydrate was synthesized at normal pressure or high pressure, the decomposition rate of calcium silicate hydrate(η(SiO2)) decreased with the reaction time postponing. But the calcium silicate hydrate formed at normal pressure appears different when Na2O is 150g/L, which might attribute to the decomposition of unstable calcium silicate hydrate and transformation into a stable form because the phenomena isnt observed when calcium silicate hydrate is synthesized at 170℃. The results also indicate that the caustic concentration favors η(SiO2), but synthesis temperature does not favor η(SiO2) because the synthesis temperature affects the structure of calcium silicate hydrate obviously, which will be discussed follows.
All η(SiO2) were less than 1.2% and the concentration of SiO2 in slurry were around 0.3g/L, which implied the stable existence of calcium silicate hydrate in caustic solution.
3.2.2 Reaction behavior of calcium silicate hydrate in soda solution
The reaction equation between calcium silicate hydrate and soda can be expressed as follows,
CaO·SiO2·nH2O+CO2-3= CaCO3+SiO2(OH)2-2+(n-1)H2O
In general, calcium carbonate is a stable compound with low solubility and is decomposed only in the concentrated caustic solution at high temperature[1]. The reaction behavior of calcium silicate hydrate in soda solution is plotted in Fig.2.
Though both of CSH-1 and CSH-2 were synthesized at normal pressure, the initial η(SiO2) appeared different The initial η(SiO2) of CSH-2 was less than that of CSH-1 but increased quickly with the reaction time till as much as that of the latter. Results indicate that all η(SiO2) increase with soda concentration but decrease with the synthesis temperature. Meanwhile η(SiO2) of CSH-1 or CSH-2 increases obviously with reaction time but CSH-3 shows different. All facts as mentioned above prove that the synthesis temperature affects the stability of calcium silicate hydrate in soda solution.
Table 1 Synthesis and composition of calcium silicate hydrate

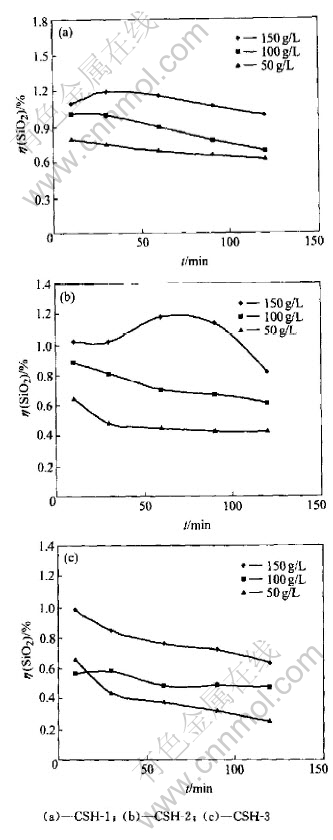
Fig.1 Effect of caustic concentration on decomposition of calcium silicate hydrate at temperature of 50℃, mass ratio of liquid to solid of 10

Fig.2 Effect of soda concentration(Na2O) on decomposition of calcium silicate hydrate at 50℃ and mass ratio of liquid to solid of 10
When calcium silicate hydrate reacted with soda solution, all η(SiO2) were more than 2%, and most of concentration of SiO2 was more than 1g/L, which meant unstable existence of calcium silicate hydrate in soda solution comparing its reaction with caustic solution. So it is necessary to decrease the soda concentration in the transformation of DSP into calcium silicate hydrate.
3.3 XRD pattern of calcium silicate hydrate
More than 30 types of calcium silicate hydrate with stable phase were reported in cement industry[7]. And sometimes CaO·SiO2·H2O(CSH(Ⅰ)) comes into being during the sinter leaching process in alumina production. And the formation conditions of CaO·SiO2·H2O, 2CaO·SiO2·0.5H2O and Na2O·2CaO·2SiO2·nH2O were studied in hydro-chemical process at high pressure, which were different from the calcium silicate hydrate in the paper, so XRD was employed to determine what kind of calcium silicate hydrate was formed and its reaction product. Fig.3 shows the XRD patterns of the calcium silicate hydrate.
The results show that when x(CaO)/x(SiO2)=1 in the synthesis of calcium silicate hydrate, the higher the temperature is, the more perfect structure calcium silicate hydrate appears. When synthesis temperature was 50℃, 3 peaks could be found in the XRD pattern (only one intensive but broad), and the main component was CaO·SiO2·H2O (CSH(Ⅰ)), which appeared bad crystal[6, 8, 14]. When the synthesis temperature is 100℃, more peaks appear, and Ca5(OH)2Si6O16·4H2O, as well asCaO·SiO2·H2O (CSH(Ⅰ) in solid residue, can be found. And then at 170℃, there appear many sharp symmetrical peaks which calcium silicate hydrate is crystallized with perfect structure, and there exist Ca6Si6O17(OH)2 and Ca5 (OH)2Si6O16·4H2O, but little CaO·SiO2·H2O is found.
Compared with the structure of calcium silicate hydrate formed in cement industry, calcium silicate hydrate in this paper also has a layer structure that contains CaO polyhedra sandwiched between two silicate chains[4, 5, 15]. The calcium sili-

Fig.3 XRD patterns of calcium silicate hydrate
cate hydrate synthesized at 50℃ has a bad structure in which the bridging tetrahedral might miss in silicate chain resulting in depolymerization and disorder, and more calcium cation and water molecule would appear in the interlayer. But calcium silicate hydrate synthesized at 170℃ showed well crystallized. All these explained why synthesized temperature affected η(SiO2) obviously whether in caustic solution or in soda solution.
When calcium silicate hydrate reacted with caustic solution , the XRD pattern of residue(Fig.4) found similar to that in Fig.3(b) because the number and the value of 2θ of the main characteristic peaks were similar. And there also exist CaO·SiO2·H2O(CSH(Ⅰ)) and Ca5(OH)2Si6O16·4H2O in both residue. All the facts prove that calcium silicate hydrate is stable in caustic solution, which is in agreement with the results from Fig.5.
Calcium silicate hydrate appears unstable in soda solution owing to the formation of CaCO3. Though Ca6Si6O17(OH)2, Ca5(OH)2Si6O16·4H2O remain in residue due to their better structure and stability, CaO·SiO2·H2O(CSH(Ⅰ)) disappears obviously for bad structure. So it is important to synthesized calcium silicate hydrate at high temperature for increasing its solubility in basic solution when DSP is to be changed into calcium silicate hydrate.
4 CONCLUSIONS
1) The composition of calcium silicate hydrate appeares different at different temperatures and x(CaO)/x(SiO2)=1, and the better structure can be found with the increase of temperature.
2) When calcium silicate hydrate reacts with caustic solution, η(SiO2) increases with temperature and caustic concentration, but decreases with the reaction time. The residue of XRD pattern after reaction has the same components of calcium silicate hydrate and η(SiO2) is low which indicates the stable existence of calcium silicate hydrate in caustic solution.
3) When reacted with soda solution, η(SiO2) increases with soda concentration and time; but decreases with synthesis temperature. More CaCO3 is formed mainly due to the decomposition of CaO·SiO2·H2O(CSH(Ⅰ)). So calcium silicate hydrate appeares unstable in soda solution.

Fig.4 XRD patterns of residue after reaction of CSH-2 with caustic solution
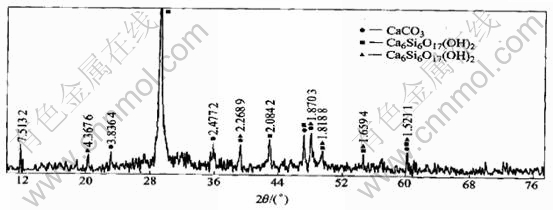
Fig.5 XRD patterns of residue after reaction of CSH-2 with soda solution
REFERENCES
[1]YANG Zhong-yu. The Technology of Alumina Production(Revised) [M]. Beijing: Metallurgical Industry Press, 1993. 1-20.(in Chinese)
[2]Sazin V S. Advance in the Treatment of Alumino-silicate Mineral or Bauxite with High Silica by Hydro-chemical Process [M]. Moskva: Metallurgy Press, 1988. 63-100.(in Russion)
[3]Abramov B Y, Nikolaev I V, Stelmakova G D. The Physico-chemical Principle for Treatment of Bauxite [M]. Moskva: Metallurgy Press, 1985. 98-129.(in Russion)
[4]CONG Xian-dong, Kirkpatrick R J. 29Si and 27O NMR investigation of the structure of some crystalline calcium silicate hydrate [J]. Advn Cem Bas Mat, 1996, 3: 133-143.
[5]CONG Xian-dong. Kirkpatrick R J. 29Si NMR study of the structure of calcium silicate hydrate [J]. Advn Cem Bas Mat, 1996, 3: 144-156.
[6]Rahman M M, Nagasaki S, Tanaka S. A model of dissolution of CaO-SiO2-H2O gel at Ca/Si>1 [J]. Cement and Concrete Research, 1999, 29: 1091-1097.
[7]Shaw S, Clark S M, Henderson C M B. Hydrothermal formation of the calcium silicate hydrates tobermorite(Ca5Si6O16(OH)2·4H2O) and xonotlite(Ca6Si6O17- (OH)2): an in situ synchrotron study [J]. Chemical Geology, 2000, 167: 129-140.
[8]Hamlin M J. Aqueous solubility relationships for two types of calcium silicate hydrate [J]. J Am Ceram Soc, 1986, 69(8): 614-618.
[9]Macphee D E, Luke K, Glasser F P, et al. Solubility and aging of calcium silicate hydrate in alkaline solution at 25℃ [J]. J Am Ceram Soc, 1989, 72(4): 646-654.
[10]Yoshihiko O, Kaori S, ZHONG Bai-qian. Formation of β-C2S by the decomposition of hydrothermally prepared C-S-H with Ca(OH)2 [J]. J Am Ceram Soc, 1994, 77(5): 1319-1323.
[11]Nocun-Wczelik W. Effect of Na and Al on the phase composition and morphology of autoclave calcium silicate hydrate [J]. Cement and Concrete Research, 1999, 29: 1759-1767.
[12]Nocun-Wczelik W. Effect of some inorganic admixtures on the formation and properties of calcium silicate hydrates product in hydrothermal conditions [J]. Cement and Concrete Research, 1997, 27(1): 83-92.
[13]Wencil B P. The system sodium oxide-calcium oxide-silicon oxide-water [J]. J Am Ceram Soc, 1990, 73(11): 3457-3461.
[14]Glasser F P, Lachowski E E, Macphee D E. Compositional model for calcium silicate hydrate(C-S-H)gels, their solubilities and free energies of formation [J]. J Am Ceram Soc, 1987, 70(7): 481-485.
[15]Babuskin V I, Matveev G M, Mchedlov-Petrocjan O P. Thermodynamics of Silicate [M]. Moskva: Metallurgy Press, 1972. 78-133.(in Russion)
(Edited by LONG Huai-zhong)
Foundation item: Project(50274076) supported by the National Natural Science Foundation of China; Project(1999064910) supported by the State Key Fundamental Research Program of China
Received date: 2004-04-01; Accepted date: 2004-07-01
Correspondence: LIU Gui-hua, PhD; Tel: +86-731-8830453