Effect of transformation pH on performance of nano-scale β-Ni(OH)2
来源期刊:中国有色金属学报(英文版)2007年第3期
论文作者:赵力 王守军 盛军 韩喜江
文章页码:558 - 558
Key words:nano material; Ni(OH)2; transformation pH; preparation
Abstract: The influence of transformation pH value on the performance of nano-scale Ni(OH)2 was analyzed. The measurement results of XRD and TEM indicate that the samples are composed of β-Ni(OH)2 with crystal size of 20-50 nm, and the crystal lattice parameters of nano-scale Ni(OH)2 prepared at different transformation pH values are different. With the increase of transformation pH value, the agglomeration of nano-scale Ni(OH)2 becomes obvious. Cyclic voltammograms(CV) and electrochemical impedance spectroscopy(EIS) measurement results show that transformation pH value affects the proton diffusion coefficient(D) and charge-transfer resistance(Rct) of the material. The specific capacity is up to 327.8 mA·h/g, and the discharge performance of electrodes depends on both D and Rct, so the kinetic characteristics that electrodes reaction is controlled by both mass-transfer step and charge-transfer step was put forward.
基金信息:the National Natural Science Foundation of China

ZHAO Li(赵 力)1, WANG Shou-jun(王守军)2, SHENG Jun(盛 军)1, HAN Xi-jiang(韩喜江)1
1. Department of Applied Chemistry, Harbin Institute of Technology, Harbin 150001,China;
2. Department of Product, DLG Battery (Shenzhen) Co. Ltd, Shenzhen 518109, China
Received 20 July 2006; accepted 27 January 2007
Abstract: The influence of transformation pH value on the performance of nano-scale Ni(OH)2 was analyzed. The measurement results of XRD and TEM indicate that the samples are composed of β-Ni(OH)2 with crystal size of 20-50 nm, and the crystal lattice parameters of nano-scale Ni(OH)2 prepared at different transformation pH values are different. With the increase of transformation pH value, the agglomeration of nano-scale Ni(OH)2 becomes obvious. Cyclic voltammograms(CV) and electrochemical impedance spectroscopy(EIS) measurement results show that transformation pH value affects the proton diffusion coefficient(D) and charge-transfer resistance(Rct) of the material. The specific capacity is up to 327.8 mA?h/g, and the discharge performance of electrodes depends on both D and Rct, so the kinetic characteristics that electrodes reaction is controlled by both mass-transfer step and charge-transfer step was put forward.
Key words: nano material; Ni(OH)2; transformation pH; preparation
1 Introduction
Nano-scale Ni(OH)2 is a new kind of battery material[1-3]. It exhibits many superior electrochemical performance, such as larger proton diffusion coefficient and higher electrochemical reaction activity, so much interest has focused on investigation of nano-scale Ni(OH)2[4-6]. HE et al[7-8] prepared nano-scale β-Ni(OH)2 by the method of controlled crystallization and granulation process, and found that nano-scale Ni(OH)2 mixed with nano-scale Co(OH)2 presents higher specific capacity, reaching its theoretical value of 289 mA?h/g at 1C, and also exhibiting much improved electrochemical performance at high discharge capacity rate up to 10C. Nanosized cathode material Ni(OH)2 was prepared by aqueous solution precipitation reaction under ultrasonic dispersing condition by ZHOU et al[9]. The nano-sized Ni(OH)2 prepared is of β-type crystal with the particle size ranging from 10 nm to 40 nm. The measured proton diffusion coefficient is 1.8×10-12 cm2/s, and the specific discharge capacity is 381 mA?h/g. WANG et al[10] found that the high density nano- crystalline multiphase nickel hydroxide is composed of a mixed phase of α-Ni(OH)2 and β-Ni(OH)2. The mechanism of the electrode reaction is still found to be controlled by proton diffusion, and the proton diffusion coefficient is 5.67×10-10 cm2/s. ZHUANG et al[11] synthesized nano-sized Ni0.96Co0.04(OH)2-C by a precipitation- transformation method, and found that substituting Ni with Co(Ni0.96Co0.04(OH)2) can upgrade nano-Ni(OH)2 rate discharge ability and electrochemical reversibility, while adding both Co and carbon black can increase discharge capacity. TIAN et al[12] prepared nickel hydroxide nanorods by using hydrothermal synthesis method.
According to Refs.[13-16], a majority of researchers have laid their emphasis on the discoveries of new preparation methods. However, there are very few detailed reports on a certain preparation method and the relationship between the preparation conditions and the corresponding performance of nano-scale Ni(OH)2, especially its electrochemical behaviour. So in this study, on the basis of the preparation of nano-scale Ni(OH)2 through precipitate transformation method, the emphasis was centralized on the influence of transformation pH value on the electrochemical performance of nano-scale Ni(OH)2, and some relative problems were discussed.
2 Experimental
2.1 Preparation of materials
0.1 mol/L Ni(NO3)2 solution was mixed with Na2C2O4 solution at 65 ℃ under stirring to yield precipitate of NiC2O4·2H2O. 10 min later, surfactant Tween-80 was added. After stirring for 10 min, the mixed solution and 0.5 mol/L NaOH solution were dropped into the starting solution (NaOH solution with certain pH values) to transform NiC2O4·2H2O into Ni(OH)2. The reaction system was stirred for 5 h, so that the transformation was fully completed. The precipitate was separated by centrifuge and rinsed with distilled water and acetone, and then it was oven-dried. After pulverized, nano-scale Ni(OH)2 was obtained. The pH value of the starting solution was controlled at 9.8, 10.1, 10.5 and 10.8, respectively.
2.2 Characterization of nano-scale Ni(OH)2
A D/MAX-A diffractometer that was made in Japan was used to measure the XRD spectrum of the samples with voltage of 35 kV, Cu Kα and scan rate of 2 (?)/min. TEM observation was performed with a 1200EX micro-scope that was made in Japan with voltage of 80 kV and Cu target. Zeta(ζ) potential was measured by using a Zetasizer 3000HS made by Malvern.
2.3 Electrochemical behaviour of nano-scale Ni(OH)2
The electrochemical behaviour of nano-scale Ni(OH)2 was investigated, and in order to estimate the electrochemical behaviour of nano-scale Ni(OH)2 in practical cell, the performance of the mixture composed of micron-scale Ni(OH)2, nano-scale Ni(OH)2, Ni powder and CoO powder was also tested.
2.3.1 Cyclic voltammograms
Cyclic voltammetric measurement was performed on CHI630a electrochemical workstation. The working electrode was a powder microelectrode[17] with a diameter of 80 μm, and the samples were filled into the powder microelectrode. The reference electrode was a Hg/HgO with OH- electrode. The electrolyte was a 7 mol/L KOH solution with 15 g/L LiOH and the scan range was 0-0.70 V.
2.3.2 Electrochemical impedance spectroscopy
EIS was measured using an EG&G PARC Model 273 Potentiostat/Galvanostat, a Model 5210 Lock-in- Amplifier and an IBM computer. The measurements were made at the open circuit potential with a superimposed 5 mV sinusoidal voltage in the frequency range of 10 mHz-10 kHz, and the experiment data were analyzed by using a nonlinear least squares(NLLS) fitting program equivalent circuit developed by Boukamp.
2.3.3 Discharge performance
The density of nano-scale Ni(OH)2 is relatively small, so it cannot be used as electrode-active material alone. Here the mixture of nano-scale Ni(OH)2 and micro-scale Ni(OH)2 was used as the active material, while Co and Ni powders were additives, and the substrate was a 1 cm×1 cm foam nickel. The counter electrode was an excessive metal hydride electrode. A single nickel electrode was sandwiched between two metal hydride electrodes and the assembly was immersed in the electrolyte consisting of 7 mol/L KOH and 15 g/L LiOH, then the simulated cell was prepared[18]. The reference electrode was a Hg/HgO with OH- electrode. After the simulate battery was activated, the discharge curves were tested.3 Results and discussion
3.1 Morphology and crystallization style
XRD and TEM results of nano-scale Ni(OH)2 are shown in Figs.1 and 2, respectively.
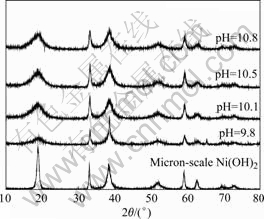
Fig.1 XRD patterns of nano-scale Ni(OH)2 prepared at different pH values
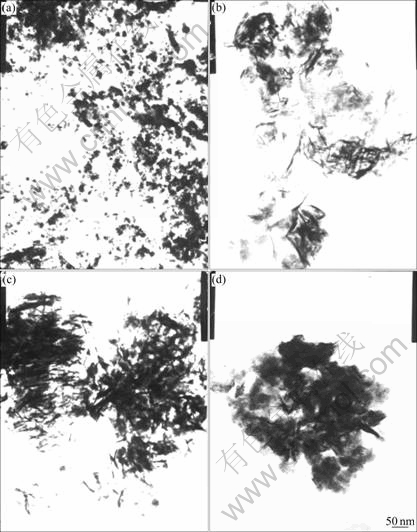
Fig.2 TEM micrographs of nano-scale Ni(OH)2 prepared at different transformation pH values: (a) pH=9.8; (b) pH=10.1; (c) pH=10.5; (d) pH=10.8
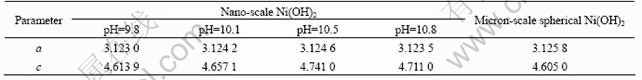
Fig.1 indicates that all the samples are typically β-phase Ni(OH)2, and some characteristic peaks of nano-scale Ni(OH)2 such as (001), (101), (102) and (111) are broadened, and this indicates that the crystal grain is refined. Table 1 lists the crystal lattice parameters of nano-scale Ni(OH)2 and micron-scale spherical Ni(OH)2 calculated from Fig.1. It can be deduced from Table 1 that the values of parameter a of all samples are almost the same, but the those of parameter c are quite different, and the sample prepared at pH of 10.5 has the maximum parameter c. The parameter c of micron-scale Ni(OH)2 is smaller than that of nano-scale spherical Ni(OH)2, and this illustrates that nano-scale Ni(OH)2 has bigger inter-lattice-layer distance than that of micron-scale Ni(OH)2, which is favorable to the diffusion of proton between two inter-layers.
Table 1 Crystal lattice parameters of samples
It can be seen from Fig.2 that the nano-scale Ni(OH)2 is in pin shape or flake shape. The agglomeration of nano-scale Ni(OH)2 becomes significant with the increase of transformation pH value. The crystallization velocity of Ni(OH)2 produced by OH- reacting with Ni2+ is different on different crystal faces, which leads to different growth velocity on different crystal faces, so the samples show an anomaly shape such as pin shape or flake shape. Nano-scale material is prone to agglomerate, owing to its characteristics. During the process of agglomeration, zeta (ζ) potential is a key impact factor. The effect of transformation pH value on ζ potential is given in Fig.3. ζ potential decreases with the increase of transformation pH value, which means that the charge number on the surface of nano-scale Ni(OH)2 diminishs when the transformation pH value is enhanced, and this makes the agglomeration of nano-scale Ni(OH)2 particles become easier. This result is in agreement with Fig.2.
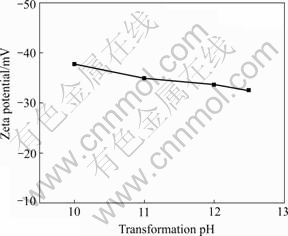
Fig.3 Effect of transformation pH value on zeta potential
3.2 Cyclic voltammetry behaviour
Cyclic voltammetric curves of nano-scale Ni(OH)2 are given in Fig.4(a). It can be seen from Fig.4(a) that the anodic peak potentials Epa of samples prepared at the pH value of 10.1, 10.5 and 10.8 are almost the same, which are lower than those of the samples prepared at the pH value of 9.8. This demonstrates that these samples will exhibit smaller polarization during the charge process. The cathodic peak potentials Epc of samples prepared at the pH values of 9.8 and 10.5 are higher than those of others, indicating that these samples give higher discharge platform. According to Epa and Epc of samples, it can be deduced that the sample prepared at the pH value of 10.5 exhibits superior electrochemical activity. Fig.4(b) shows the cyclic voltammetric curves of mixtures. It has the following characteristics: 1) The Epa and Epc of all mixtures become low compared with Fig.4(a); 2) The peak current increases obviously; 3) The Epa of the samples prepared at the pH value of 9.8 and 10.8 is lower than that of others, but the sample prepared at a pH value of 10.1 has the highest Epc, indicating that the sample prepared at a pH value of 10.1 exhibits superior discharge performance.
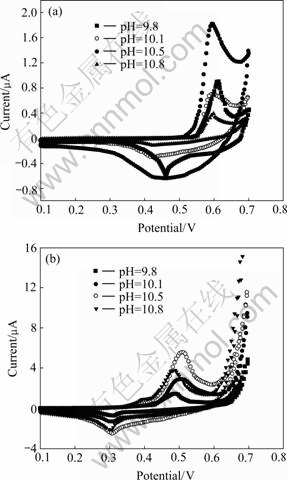
Fig.4 Cyclic voltammograms of samples: (a) Nano-scale Ni(OH)2; (b) Mixtures
We can estimate the proton diffusion coefficient (D) of Ni(OH)2 by using the following formula:
Ip=2.69×105×n3/2×A×(Dv)1/2×C0 (1)
where Ip is the current of peak, A; n is the transfer electron number; A is the area of electrode, cm2; D is the proton diffusion coefficient, cm2/S; v is the scan rate, V/s; C0 is the concentration of reactant, mol/mL.
By changing scan rate, the corresponding Ip was measured, and Ip—v1/2 curve was gotten. From the slope of Ip—v1/2 curve, the proton diffusion coefficient of Ni(OH)2 was estimated. The calculation results are listed in Table 2.
The data show that, with the increase of transforma- tion pH value, the proton diffusion coefficient increases firstly, and reaches its maximum at the pH value of 10.5, then decreases. The increase of proton diffusion coefficient can accelerate the rate of proton diffusion in Ni(OH)2, decrease the concentration polarization, and enhance the electrochemical performance of Ni(OH)2. The proton diffusion coefficient is relative to c value of Ni(OH)2 crystal lattice. It can be concluded from Table 1 that the increase of c value leads to the increase of the inter-layer distance, which also means a smaller inter-layer proton diffusion resistance and a bigger proton diffusion coefficient. This result is in agreement with Table 1. After additives are added in nano-scale Ni(OH)2, the proton diffusion coefficient decreases significantly. This may be due to the fact that the addition of other additives makes the contact between Ni(OH)2 particles become poor, and the diffusion of proton becomes difficult.
Table 2 Effects of transformation pH value on proton diffusion coefficient of samples (10-14cm2?s-1)

3.3 Electrochemical impedance performance of samples
In order to estimate the performance of nano-scale Ni(OH)2 in practical batteries, the EIS research is focused on the mixtures. The complex plane plots of mixtures are given in Fig.5, and the fitting results of charge-transfer resistance (Rct) are listed in Table 3. It is obvious that different mixtures exhibit different Rct, and there is no distinct regular relationship between Rct and transformation pH value. It can be seen from Table 3 that the Rct of mixtures is relatively small when the pH value equals 10.1 and 10.8, indicating that the charge-transfer process of these mixtures is easier.

Fig.5 Nyquist plots of different mixtures
Table 3 Charge-transfer resistance of mixtures (Ω)
![]()
In other words, the electrochemical polarization of these mixtures in the charge-discharge process is lower than that of others, which is beneficial to the improvement of electrode performance.
3.4 Discharge performance
The discharge curves of samples are given in Fig.6. It can be seen that different samples exhibit different specific capacities, and the sample prepared at the pH of 10.1 gives the maximum specific capacity (327.8 mA?h/g) and higher discharge platform. In Table 2, it can be found that the sample prepared at the pH value of 10.5 exhibits the maximum proton diffusion coefficient, which means that the concentration polarization of this sample is relatively small in the process of charge-discharge. From Table 3, it can be seen that the sample prepared under the pH value of 10.8 shows the relatively small charge-transfer resistance. But the discharge results indicate that the discharge performance of these two samples is inferior to that of the other samples. This is because that the discharge performance of Ni(OH)2 electrodes is not decided by one factor (Rct or D), but depends on two factors: Rct and D. The sample prepared at the pH value of 10.1 has suitable Rct and D (relatively small Rct and relatively large D), so it exhibits higher discharge platform and larger specific capacity. This means that the whole anode reaction is not controlled by the single mass-transfer step or the charge-transfer step, but by both of them collectively.
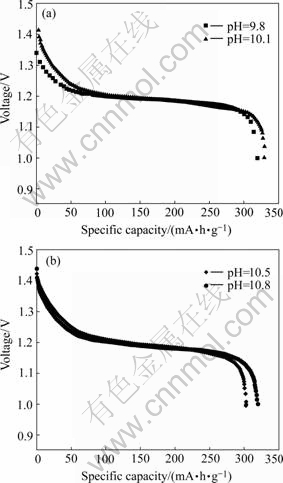
Fig.6 Discharge curves of samples prepared under different pH values
4 Conclusions
1) Transformation pH value exerts a great influence on the agglomeration degree of nano-scale Ni(OH)2. With the increase of transformation pH value, the agglomeration of nano-scale Ni(OH)2 becomes more obvious, but all samples are β-type Ni(OH)2.
2) Samples prepared at different transformation pH exhibit different D and Rct, and the samples prepared with the transformation pH equal to 10.5 and 10.1 have the maximal D and minimal Rct, respectively.
3) The discharge performance of electrodes depends on D and Rct, so the kinetic characteristics of electrodes reaction are controlled by both mass-transfer step and charge-transfer step.
References
[1] DAVID E R, ALVIN J S, PETER R S, XIAO T D. Nickel hydroxide and other nanophase cathode materials for rechargeable batteries [J]. Journal of Power Sources, 1997, 65: 231-233.
[2] KONSTANTINOV K, ZHONG S, WANG CY, LIU H K, DOU S X. Fabrication and properties of spray-dried nanofeatured spherical Ni(OH)2 materials [J]. Journal of Nanoscience and Nanotechnology, 2002, 2(6): 675-678.
[3] XIAN Y W, HEAN L, PARKHUTIK P V, MILLAN A C. Study of the performance of nanostructural multiphase nickel hydroxide [J]. Journal of Power Sources, 2003, 115: 153-160.
[4] XIAO T S D, STRUTT P K, KEAR B H, CHEN H M, WANG D M. Nanostructured oxides and hydroxides and methods of synthesis therefore [P]. US 6162530, 2000.
[5] YANG D N, WANG R M, ZHANG J, LIU Z. Synthesis of nickel hydroxide nanoribbons with a new phase: A solution chemistry approach [J]. Journal of Physical Chemistry B, 2004, 108(23): 7531-7533.
[6] LIU Chang-jiu, YE Nai-qing, DIAO Han-ming. Preparation and electrochemical behavior of electrode composed of nano β-Ni(OH)2 and NiO [J]. Chinese Journal of Applied Chemistry, 2001,18(4): 335-337. (in Chinese)
[7] HE X M, LI J J, CHENG H W, JIANG C Y, WAN C R. Controlled crystallization and granulation of nano-scale β-Ni(OH)2 cathode materials for high power Ni-MH batteries [J]. Journal of Power Sources, 2005, 152: 285-290.
[8] HE Xiang-ming, LI Jian-jun, CHENG Hong-wei, JIANG Chang-yin, WAN Chun-rong. Preparation of nano-scale Ni(OH)2 based on controlled crystallization [J]. Journal of Inorganic Materials, 2005, 20(6): 1317-1321. (in Chinese)
[9] ZHOU H B, ZHOU Z T. Preparation, structure and electrochemical performances of nanosized cathode active material Ni(OH)2 [J]. Solid State Ionics, 2005, 176: 1909-1914.
[10] WANG X Y, SEBASTIAN P J, MILLAN A C, PARKHUTIK P V, SEBASTIAN P J, GAMBOA S A. Electrochemical study of nanostructured multiphase nickel hydroxide [J]. Journal of New Materials for Electrochemical Systems. 2005, 8(2): 101-108.
[11] ZHUANG Xiao-gue, CHENG Jie, YANG Dong-ping, CAO Gao-ping, YANG Yu-sheng. Structure and electrochemical characters of nano-sized Ni(OH)2-C prepared by precipitation-transformation method [J]. Journal of Inorganic Materials, 2005, 20(2): 337-344. (in Chinese)
[12] TIAN Zhou-ling, JIAO Qing-ze, ZHAO Yun. Preparation and characterization of nickel hydroxide nanorods using hydrothermal synthesis method [J]. Chemical Journal of Chinese Universities, 2005, 26(8): 1387-1390. (in Chinese)
[13] YAN Shao-ping. Fabrication and characterization of nickel hydroxide nanoparticles by W/O type microemulsion and reversed micelle [J]. Physics, 2002, 31(4): 246-248. (in Chinese)
[14] MEYER M, BEE A, TALBOT D, CABUIL V. Synthesis and dispersion of Ni(OH)2 platelet-like nanoparticles in water [J]. Journal of Colloid and Interface Science, 2004, 277(2): 309-315.
[15] LIU X H, YU L. Synthesis of nanosized nickel hydroxide by solid- state reaction at room temperature [J]. Materials Letters, 2004, 58: 1327-1330.
[16] JEEVANANDAM P, KOLTYPIN Y, GEDANKEN A. Synthesis of nanosized alpha-nickel hydroxide by a sonochemical method [J].Nano Letters, 2001, 1(5): 263-266.
[17] CHA C S, LI C M, YANG H X, LIU P F. Powder microelectrodes [J]. Jornal of Electroanalytical Chemistry, 1994, 368: 47-54.
[18] LIU Yuan-gang, TANG Zhi-yuan, XU Qiang, ZHANG Xiao-yang, LIU Yong. Ni(OH)2 particles synthesized by high energy ball milling [J]. Trans Nonferrous Met Soc China, 2006, 16(5): 1218-1222.
Foundation item: Project(20271015) supported by the National Natural Science Foundation of China
Corresponding author: ZHAO Li; Tel: +86-451-86413721; E-mail: dhx907@hit.edu.cn

