文章编号: 1004-0609(2006)06-1120-05
淬冷法研究NbCl5-NaCl和
NbCl5-KCl二元系的热力学性质
李军丽, 杨冬梅, 曾繁武, 王之昌
(东北大学 理学院, 沈阳 110004)
摘 要: 用高温平衡-淬冷方法, 研究了NaCl-NbCl5和KCl-NbCl5二元系的热力学性质。 结果表明: 在580~680K和0.06~0.12MPa范围内, NaNbCl6(g)和KNbCl6(g)分别是惟一产物, 且NaNbCl6(g)比KNbCl6(g)稳定。 反应NbCl5 (g)+NaCl (s)-NaNbCl6 (g)的热力学函数是 和
和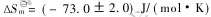 ; 反应NbCl5 (g)+KCl (s)-KNbCl6 (g)的热力学函数是
; 反应NbCl5 (g)+KCl (s)-KNbCl6 (g)的热力学函数是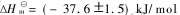 和
和 。
。
关键词: NaNbCl6; KNbCl6; NbCl5; NaCl; KCl; 气态配合物; 淬冷法 中图分类号: O642
文献标识码: A
Thermodynamic properties of NbCl5-NaCl and
NbCl5-KCl binary systems by quenching experiments
LI Jun-li, YANG Dong-mei, ZENG Fan-wu, WANG Zhi-chang
(School of Sciences, Northeastern University, Shenyang 110004, China)
Abstract: Thermodynamic properties of NaCl-NbCl5 and KCl-NbCl5 binary systems were studied by high temperature equilibrated quenching experiments. The results show that NaNbCl6(g) and KNbCl6(g) complexes are respectively their sole predominant vapour complexes roughly in the ranges of 580-680K and 0.06-0.12MPa, and that NaNbCl6(g) is more stable than KNbCl6(g). The thermodynamic properties are and
and 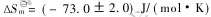 for the reaction NbCl5 (g)+NaCl (s)-NaNbCl6 (g), and
for the reaction NbCl5 (g)+NaCl (s)-NaNbCl6 (g), and 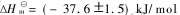 and
and  for the reaction NbCl5 (g)+KCl (s)-KNbCl6 (g).
for the reaction NbCl5 (g)+KCl (s)-KNbCl6 (g).
Key words: NaNbCl6; KNbCl6; NbCl5; NaCl; KCl; vapour complex; quenching method
在自然界中, 铌总是和其它金属元素在矿物中共生, 其中, 具有工业价值的矿物有铌铁矿等。 我国的白云鄂博特大型共生矿中, 不仅富含铁和稀土元素, 也有大量铌。 氯化法是从矿物中提取和分离铌的重要方法之一[1-4]。 在氯化反应中, 矿物中的铌、 铁等元素都生成气态产物, 随后的铌-铁和铌-钽等分离工作通常在盐浴中进行。 因此, NbCl5-NaCl和NbCl5-KCl二元系的热力学性质十分重要。 Sadoway和Flengas等[5, 6]曾经用蒸气压等方法研究NbCl5-NaCl和NbCl5-KCl二元系中固态和液态配合物NaNbCl6(s, l)和KNbCl6(s, l)的热力学性质; 另一方面, Schfer[7]曾经指出NbCl5-NaCl二元系中生成气态配合物的可能性; 但是, 文献中没有NbCl5-NaCl和NbCl5-KCl二元系中气态配合物的热力学性质的报道。
本文作者[8-15]曾经用高温平衡-淬冷法研究LnCl3-AlCl3(Ln=稀土元素)等二元系的热力学性质。 本文报道NbCl5-NaCl和NbCl5-KCl二元系的热力学性质的高温平衡-淬冷法实验研究结果。
1 实验
1.1 实验材料
NbCl5纯度是99.9%(质量分数)(美国Aldrich公司)。 NaCl、 KCl为分析纯试剂, 用二次蒸馏水两次重结晶以后, 经加热真空干燥处理。 实验用反应瓶由Pyrex玻璃制作, 如图1所示, 体积为50~60mL。
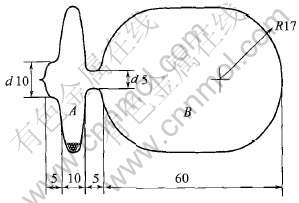
图1 反应瓶示意图
Fig.1 Schematic diagram of ampoule(mm)
1.2 淬冷实验
采用类似于文献[8-15]中的高温平衡-淬冷实验方法。 首先, 在氩气手套箱内, 称取适量的NaCl和NbCl5或KCl和NbCl5, 在玛瑙研钵中混匀研磨, 加入反应瓶A端(图1); 经反复抽真空和纯氩气洗净后, 将反应瓶抽空密封。 然后, 将4个装有不同数量的NbCl5和NaCl(或NbCl5和KCl)的反应瓶, 置于反应炉恒温区的石墨容器(图2)内, 因此, 每一个二元系在同一个反应温度有4个平衡压力不同的试样。 通过预备实验, 考察了反应时间对反应平衡常数的影响。 结果表明: 反应在5h 内达到平衡(反应5h 后, 平衡常数不随时间的增加而改变), 因此, 每一个反应的实际平衡时间取为6 h。 每次反应温度恒定范围不超过±2K。 达到平衡后, 迅速将石墨容器从反应炉中取出, 并立即在每个反应瓶上盖上潮湿的石棉布, 并用冷水淋洗, 使气相平衡物迅速、 均匀地沉积在反应瓶内壁。 最后, 用化学分析方法测定反应瓶B端(图1)沉积物中的离子Nb5+和Na+或离子Nb5+和K+的摩尔数, 并用蒸馏水滴定的方法测得反应瓶B端的体积。
相图[16]显示: NaCl-NbCl5二元系在温度T≥703K时和KCl-NbCl5二元系在温度T≥638K时, 液、 固相有可能同时存在, 使组元活度无法确定。 此外, 据文献[6]报道, 固态配合物NaNbCl6在518K和572K时分别有α→β相变和β→γ相变, 固态配合物KNbCl6在572K有α→β相变。 因此, 本文选择NaCl-NbCl5二元系的实验在588~673K范围内进行, KCl-NbCl5二元系的实验在583~628K范围内进行, 此时NbCl5全部为气态, NaCl、 KCl为纯固态, 活度都等于1; 此外, 固态配合物NaNbCl6和KNbCl6没有相变(尽管后者对于本实验并非决定性因素)。 此外, 当平衡压力大于0.25MPa 时, 反应瓶淬冷时易爆裂; 故选择本实验的压力范围为0.06~0.12MPa。
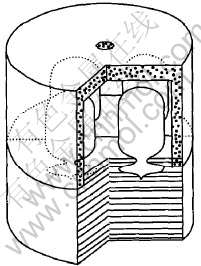
图2 石墨容器示意图
Fig.2 Schematic diagram of graphite container
1.3 化学分析
反应瓶B端沉积物中Nb5+的含量用氯代磺酚S分光光度法测定, Na+和K+的含量用原子吸收分光光度法测得。
2 结果与讨论
在本实验温度范围内, NaCl和KCl为不挥发的固态。 但是, 本实验发现在反应瓶B端沉积物中存在Na+和K+, 因此, 类似于文献[7], 可以确定有气态配合物NavNbClv+5(g)和KvNbClv+5(g)的生成。 由于文献[5, 6]中只报道1∶1型固态和液态配合物的存在, 于是可以合理地假定, 本实验中存在于反应瓶B端沉积物中的Na+和K+, 全部来源于下述反应生成的气态配合物NaNbCl6和KNbCl6:

令xc和x(NbCl5)分别表示反应瓶B端沉积物中MNbCl6(g) 和NbCl5(g) 的摩尔分数, x(Nb5+)表示沉积物中Nb5+的总量, V为反应瓶B端的体积。 有
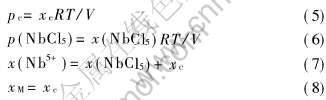
于是, 由Na+、 K+和Nb5+的化学分析结果, 通过上述各关系式, 可以求得每一温度下各样品的pc、 p(NbCl5)和Kp值。 在此情况下, 由式(3)~(8)计算出的同一体系的ln(pc/p)-ln[p(NbCl5)/p]关系和ln Kp-1/T关系都应是直线。
其次, 令c(v)表示气态配合物MvNbClv+5, 如果不仅有MNbCl6而且有M2NbCl7, M3NbCl8, ……存在, 反应(1)和(2)应改写成

因此式(7)和(8)可分别改成:
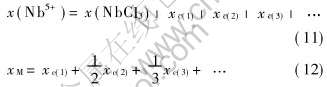
在此情况下, 类似于文献[9-11]中LnAl3Cl12和LnAl4Cl16(Ln=La和Ce)之间以及LnAl2Cl9和LnAl3Cl12(Ln=Dy和Ho)之间不同的Kp值和ln Kp-1/T关系, MNbCl6、 M2NbCl7、 M3NbCl8、 ……之间也应有不同的ln Kp-1/T关系, 于是, 由M+和Nb5+的化学分析结果, 代入式(3)~(8), 计算出表观的ln Kp-1/T关系将呈现非线性。
图3和图4分别为NbCl5-NaCl和NbCl5-KCl二元系在4个反应温度下的ln(pc/ )-ln[p(NbCl5)/
)-ln[p(NbCl5)/  ]关系, 均得到斜率相同的直线。 图5和图6分别显示了这两个二元系的Rln Kp-1/T也呈现线性关系, 从而进一步证实, 在本实验条件下, 气态配合物NaNbCl6和KNbCl6确实分别是NaCl与NbCl5之间和KCl与NbCl5之间的惟一反应产物。 图5和图6还显示, 同一温度下反应(1)的热力学平衡常数大于反应(2), 即气态配合物NaNbCl6比KNbCl6稳定。
]关系, 均得到斜率相同的直线。 图5和图6分别显示了这两个二元系的Rln Kp-1/T也呈现线性关系, 从而进一步证实, 在本实验条件下, 气态配合物NaNbCl6和KNbCl6确实分别是NaCl与NbCl5之间和KCl与NbCl5之间的惟一反应产物。 图5和图6还显示, 同一温度下反应(1)的热力学平衡常数大于反应(2), 即气态配合物NaNbCl6比KNbCl6稳定。
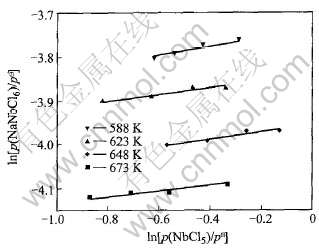
图3 NaCl-NbCl5二元系ln(pc/ )与ln[p(NbCl5)/
)与ln[p(NbCl5)/  ]的关系
]的关系
Fig.3 Relationship between ln(pc/ ) and ln[p(NbCl5)/
) and ln[p(NbCl5)/  ] for NaCl-NbCl5 binary system
] for NaCl-NbCl5 binary system
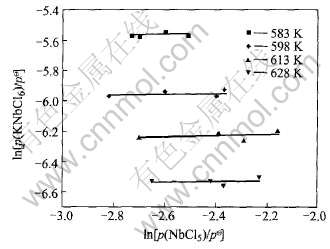
图4 KCl-NbCl5二元系ln(pc/ )与ln[p(NbCl5)/
)与ln[p(NbCl5)/  ]的关系
]的关系
Fig.4 Relation between ln(pc/ ) and ln[p(NbCl5)/
) and ln[p(NbCl5)/  ] for KCl-NbCl5 binary system
] for KCl-NbCl5 binary system

图5 NaCl-NbCl5二元系Rln Kp与1/T的关系
Fig.5 Relationship between Rln Kp and 1/T for NaCl-NbCl5 binary system
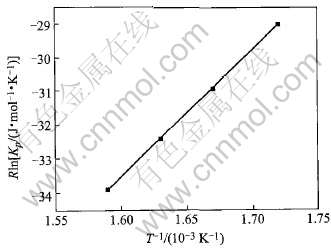
图6 KCl-NbCl5二元系Rln Kp与1/T的关系
Fig.6 Relationship between Rln Kp and 1/T for KCl-NbCl5 binary system
这两个体系的lnKp和1/T按式(13)进行最小二乘法回归分析, 得到平均反应温度Tm=633K时反应(1)和Tm=606K时反应(2)的焓变与熵变:

平衡反应(1)的焓变与熵变分别为 和
和 ; 平衡反应(2)的焓变与熵变分别为
; 平衡反应(2)的焓变与熵变分别为 和
和 。
。 和
和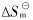 的误差分别来源于统计误差和测定误差[8-15], 其中, 后者包括反应温度误差(不大于±2K)、 反应瓶B端体积的分析误差(不大于±0.5%)、 反应瓶B端沉积物中离子Nb5+、 Na+和K+的化学分析误差(不大于±5 %)等。 所有这些因素加起来, 对
的误差分别来源于统计误差和测定误差[8-15], 其中, 后者包括反应温度误差(不大于±2K)、 反应瓶B端体积的分析误差(不大于±0.5%)、 反应瓶B端沉积物中离子Nb5+、 Na+和K+的化学分析误差(不大于±5 %)等。 所有这些因素加起来, 对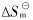 产生的总误差不会超过±2.0kJ/mol, 对
产生的总误差不会超过±2.0kJ/mol, 对 产生的总误差不会超过±1.5J/(mol·K)。
产生的总误差不会超过±1.5J/(mol·K)。
3 结论
在温度588~673K和压力0.06~0.12MPa范围内, 反应NbCl5(g)+NaCl(s)-NaNbCl6(g)的产物是NaNbCl6(g), 反应的热力学函数是 (
( , NaNbCl6(g))=(-28.3±1.5) kJ/mol 和
, NaNbCl6(g))=(-28.3±1.5) kJ/mol 和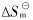 (
( , NaNbCl6(g))=(-73.0±2.0) J/(mol·K)。 在温度583~628K和压力0.06~0.12MPa范围内, 反应NbCl5(g)+KCl(s)-KNbCl6(g)的惟一产物是KNbCl6(g), 反应的热力学函数是
, NaNbCl6(g))=(-73.0±2.0) J/(mol·K)。 在温度583~628K和压力0.06~0.12MPa范围内, 反应NbCl5(g)+KCl(s)-KNbCl6(g)的惟一产物是KNbCl6(g), 反应的热力学函数是 (
( , KNbCl6(g))=(-37.6±1.5) kJ/mol和
, KNbCl6(g))=(-37.6±1.5) kJ/mol和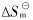 (
( , KNbCl6(g))=(-93.7±2.0) J/(mol·K)。
, KNbCl6(g))=(-93.7±2.0) J/(mol·K)。
REFERENCES
[1]Gaballah J, Allain E, Djona M. Extraction of tantalum and niobium from tin slags by chlorination and carbochlorination[J]. Metall Mater Trans B, 1997, 28B: 359-369.
[2]Sadoway D R, Flengas S N. A new process for the separation of tantalum from niobium[J]. Metall Trans B, 1980, 8B: 57-62.
[3]Sato N, Nanjo M. Separation of niobium from ferroniobium by chlorination[J]. Metall Trans B, 1985, 16B: 639-644.
[4]有色金属提取冶金手册编辑委员会. 有色金属提取冶金手册[M]. 北京: 冶金工业出版社, 2002. 211.
Editorial Board for the Handbook of Extractive Metallurgy of Nonferrous Metals. A Handbook for Extractive Metallurgy of Nonferrous Metals[M]. Beijing: Metallurgical Industry Press, 2002. 211.
[5]Sadoway D R, Flengas S N. The synthesis and properties of the hexachloroniobates and hexachlorotantalates of Na, K, Rb, and Cs[J]. Can J Chem, 1978, 56: 2013-2018.
[6]Sadoway D R, Flengas S N. Thermal properties of the alkali metal hexachloroniobate and hexachlorotantalate compounds by vapour measurements[J]. Can J Chem, 1978, 56: 2538-2545.
[7]Schfer H. Gaseous chloride complexes with halogen bridges─homo-complexes and hetero-complexes[J]. Angew Chem Int Ed Engl, 1976, 15: 713-727.
[8]Wang Z C, Wang L S, Gao R J, et al. Thermodynamic study of the rare earth vapour complexes: ScAl2Cl9 and YAl2Cl9[J]. J Chem Soc Faraday Trans, 1996, 92(11): 1887-1890.
[9]Wang L S, Gao R J, Su Y, et al. Formation thermodynamics of the rare earth vapour complexes: DyAl3Cl12 and DyAl2Cl9[J]. J Chem Thermody, 1996, 28: 1093-1102.
[10]Wang Z C, Wang L S. Thermodynamic properties of the rare earth element vapor complexes LnAl3Cl12 from Ln=La to Ln =Lu[J]. Inorg Chem, 1997, 36(8): 1536-1540.
[11]Wang Z C, Wang L S. Thermodynamic properties of the rare earth element vapor complexes LaAl4Cl15, CeAl4Cl15 and HoAl2Cl9[J]. J Alloys Comp, 1998, 265: 153-159.
[12]于锦, 杨冬梅, 蒋军辉, 等. 稀土气态配合物NdAl3Br12的热力学性质[J]. 中国有色金属学报, 2004, 14(4): 702-705.
YU Jin, YANG Dong-mei, JIANG Jun-hui, et al. Thermodynamic properties of the rare earth vapour complex NdAl3Br12[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 702-705.
[13]于锦, 李军丽, 杨冬梅, 等. FeCl3和Fe2Cl6与KCl反应的热力学研究[J]. 科学技术与工程, 2004, 4(12): 1003-1006.
YU Jin, LI Jun-li, YANG Dong-mei, et al. Thermodynamic study on the reactions of FeCl3 and Fe2Cl6 with KCl[J]. Scientific Technology and Engineering, 2004, 4(12): 1003-1006.
[14]于锦, 王林山, 杨冬梅, 等. 稀土气态配合物GdAl3Br12的热力学研究[J]. 中国稀土学报, 2004, 22(3): 326-330.
YU Jin, WANG Lin-shan, YANG Dong-mei, et al. Thermodynamic study on the rare earth vapour complex GdAl3Br12[J]. Journal of The Chinese Rare Earth Society, 2004, 22(3): 326-330.
[15]于锦, 杨冬梅, 王之昌. 稀土气态配合物PrAl3Br12的热力学性质[J]. 东北大学学报(自然科学版), 2005, 26(2): 201-204.
YU Jin, YANG Dong-mei, WANG Zhi-chang. Thermodynamic properties of the rare earth vapour complex PrAl3Br12[J]. Journal of Northeastern University (Natural Science), 2005, 26(2): 201-204.
[16]Levin E M, Robbins C R, McMurdie H F. Phase Diagrams for Ceramists[M]. Columbus: The American Ceramic Society, 1969. 342.
基金项目:国家自然科学基金资助项目(50274027)
收稿日期:2005-11-10;修订日期:2006-02-06
通讯作者:李军丽;电话:13504051630;E-mail:lijunli78@163.com
(编辑陈爱华)