漂浮阳极泥中铋的提取与三氧化二铋的制备
白猛1,郑雅杰1,洪波1,张博亚2,张传福1
(1. 中南大学 冶金科学与工程学院,湖南 长沙,410083;
2. 云南铜业股份公司冶炼加工总厂,云南 昆明,650102)
摘要:漂浮阳极泥经过盐酸浸出、稀释水解、氢氧化钠中和得到氯氧铋,氯氧铋经过氢氧化钠转化制备得到三氧化二铋。当盐酸浓度为6 mol/L,固液比为1:5,反应温度为80 ℃,反应时间为1 h时,漂浮阳极泥中锑和铋浸出率分别达到99.17%和99.08%。当稀释比为8:1时,盐酸浸出液中锑水解率为98.13%,铋水解率仅为8.8%。稀释后液中加入氢氧化钠溶液,当pH为1.5时,铋水解率达到99.5%,水解产物氯氧铋(BiOCl)中铋、氧、氯的质量分数分别为54.23%,19.30%和14.61%。氯氧铋再次经过盐酸浸出,稀释水解,氢氧化钠沉淀得到氯氧铋。除杂后氯氧铋经过硫酸洗涤、氢氧化钠转化,当氢氧化钠浓度为6 mol/L,液固比为3:1,反应温度为80 ℃时,反应 2 h后过滤,用0.5 mol/L盐酸洗涤得到形貌为纤维状、晶型为单斜的α-Bi2O3,氧化铋纯度达到99.81%。
关键词:漂浮阳极泥;浸出;铋;氧化铋
中图分类号:TF817 文献标志码:A 文章编号:1672-7207(2012)05-1622-06
Extraction of bismuth and preparation of bismuth trioxide from floating anode slime
BAI Meng1, ZHENG Ya-jie1, HONG Bo1, ZHANG Bo-ya2, ZHANG Chuan-fu1
(1. School of Metallurgy Science and Engineering, Central South University, Changsha 410083, China;
2. Yunnan Copper Company Limited, Kunming 650102, China)
Abstract: BiOCl was prepared from floating anode slime by hydrochloric acid leaching, diluting and NaOH neutralizing. BiOCl was turned into Bi2O3 by NaOH. The leaching rates of Sb and Bi are 99.17% and 99.08% when hydrochloric acid concentration is 6mol/L, reaction temperature is 80 ℃, ratio of solid to liquid is 1:5, and reaction time is 1 h. The hydrolysis rate of Sb is 98.13% and the hydrolysis rate of Bi is only 8.8% after the hydrochloric acid leaching when solution is diluted 8 times by water. The hydrolysis rate of Bi is up to 99.5% and the hydrolysis product is BiOCl in which the contents of Bi, O and Cl are respectively 54.23%, 19.30% and 14.61% after the diluted solution was adjusted to pH=1.5 by NaOH. The impurity in the BiOCl is removed after hydrochloric acid leaching, diluting and NaOH neutralizing. BiOCl is washed by sulfuric acid and treated by NaOH after removing impurity. Bi2O3 is prepared when NaOH concentration is 6 mol/L, reaction temperature is 80 ℃, ratio of liquid to solid is 3:1, and reaction time is 2 h. Bi2O3 is monoclinic and fibrous and the purity of Bi2O3 is 99.81% when Bi2O3 is washed by 0.5 mol/L hydrochloric acid.
Key words: floating anode slime; leaching; bismuth; Bi2O3
铜电解精炼时,阳极中贵金属、其他金属如硒、碲、铜及阳极溶解后形成的砷、锑、铋化合物沉降在电解槽中,这类不溶物称之为阳极泥。当这类不溶物细小成为絮状物质时,由于其表面积大、密度小而悬浮在电解液中,形成所谓“漂浮阳极泥”。漂浮阳极 泥[1]与铜阳极泥成分[2]相差较大,阳极泥中成分(质量分数)为:1%~28% Ag,0.1%~1.5% Au,0.1%~5% As,0.1%~10% Sb,0.1%~1% Bi[3-6];而漂浮阳极泥中成分为:0.04%~2% Ag,<0.1% Au,11.9%~18% As,29.5%~48.5% Sb,2%~10% Bi。漂浮阳极泥中As,Sb和Bi含量高,贵金属金、银含量低。因此,漂浮阳极泥不利于直接回收贵金属。目前,对漂浮阳极泥的湿法研究报道较少,一般将漂浮阳极泥返回熔炼炉进行火法处理。其主要缺点是造成砷、锑、铋等杂质的累积,同时产生金、银的损失。另一种是少量与阳极泥参混作为贵金属原料,提取金银。其主要缺点是由于漂浮阳极泥中砷、锑、铋含量高,处理时脱砷困难,环境污染较大[7-8]。本文作者采用盐酸浸取漂浮阳极 泥[9],使漂浮阳极泥金、银得到富集的同时,从盐酸浸取液制备得到三氧化二铋,使铋得到回收。
1 实验
1.1 实验原料
漂浮阳极泥原料成分分析结果如表1所示。
表1 漂浮阳极泥成分(质量分数)
Table 1 Composition of floating anode slime %

由表1可知:漂浮阳极泥的主要成分为As,Sb和Bi,其质量分数分别为13.83%,32.67%和9.48%。
1.2 实验步骤
将漂浮阳极泥加入到盐酸溶液中,启动搅拌,在适宜的温度下反应一定时间后过滤得到酸浸液和浸出后的漂浮阳极泥。在酸浸液中加水稀释后进行水解,水解后过滤得到锑渣和滤液,在滤液中加入氢氧化钠调节pH后过滤得到氯氧铋。
氯氧铋再次经过盐酸溶解过滤,稀释水解除杂,氢氧化钠沉淀与转化制备得到三氧化二铋,其工艺流程如图1所示。
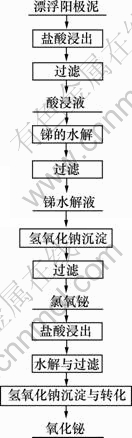
图1 漂浮阳极泥处理工艺流程图
Fig.1 Process of treatment of floating anode slime
1.3 分析方法
采用硫酸铈滴定法测定Sb3+,采用硫酸联胺还原-硫酸铈滴定法测定Sb5+[10]。溶液中As,Bi,Ag和Au等其他元素采用ICP(IRIS Intrepid Ⅱ XSP)分析。固体成分采用X荧光分析仪(PW2424)分析。固体物相采用X线衍射仪(D/max-rA,日本理学)分析。
2 结果与讨论
2.1 漂浮阳极泥的盐酸浸取
根据研究[9],实验取漂浮阳极泥1 kg,当盐酸浓度为6 mol/L,固液比为1:5,反应温度为80 ℃,反应时间为1 h时,浸出后过滤得到盐酸浸取液4.65 L。
经分析浸取液中锑质量浓度为69.67 g/L,铋质量浓度为20.20 g/L,其锑和铋浸出率分别为99.17%和99.08%。浸出后漂浮阳极泥中金含量为0.26%,银含量为13.12%,与表1相比,金银富集15倍以上。
漂浮阳极泥中金银主要以单质、硒化物和碲化物存在,盐酸浸出过程中,金、银溶解较少得到富集。铋在漂浮阳极泥中主要以BiAsO4形式存在,盐酸浸出过程中主要反应为:
BiAsO4+3HCl=BiCl3+H3AsO4 (1)
漂浮阳极泥中锑的存在形态较复杂,主要以SbAsO4形式存在,五价锑以锑酸盐、砷锑酸盐形式存在。随着SbAsO4的浸出,难溶的锑酸盐、砷锑酸盐逐步溶解。SbAsO4浸出反应如下:
SbAsO4+3HCl=SbCl3+H3AsO4 (2)
2.2 盐酸浸取液中锑和铋的分离
从溶液中分离锑和铋的方法主要有:硫化沉淀法和水解法[11-12]。水解法分离锑和铋的原理是锑的水解pH低于铋水解pH,可以通过稀释实现锑和铋的分离。
当温度为20 ℃,反应时间为1 h时,稀释比对锑和铋水解率的影响如图2所示。
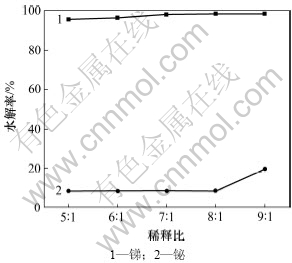
图2 稀释比对锑和铋水解率的影响
Fig.2 Effect of dilution ratio on hydrolysis rate of Sb and Bi
由图2可知:随着稀释比的增加,锑、铋水解率增大。稀释比从5:1增加到9:1,锑水解率从95.4%增加到98.3%。当稀释比低于8:1时,铋水解率在8.8%左右;当稀释比为9:1时,铋水解率增加到19.7%;当稀释比为8:1时,锑水解率为98.13%,铋水解率为8.8%,锑的水解率较大,铋的损失率较少。故适宜的水解稀释比为8:1。
稀释比为8:1时,水解产物锑渣中锑、氯、氧、砷质量分数分别为50.00%,19.02%,14.50%和13.16%,铋仅为0.16%。说明锑渣成分主要由氯、氧、锑、砷4种元素组成,铋含量低,从而实现了锑铋分离。锑渣经过洗涤除砷后,可以用来制备纯度较高的氧化锑。
溶液中五价锑水解生成非晶态锑氧化合物[5],水解过程中,三价锑的水解产生沉淀会引起五价锑的水解共沉淀,反过来五价锑的水解又会促使三价锑的水解共沉淀,三价锑与五价锑水解同时进行。
SbCl3水解产物依次有SbOCl,Sb4O5Cl2,Sb(OH)3,Sb2O3等,Sb3+在溶液中能与Cl-和OH-形成配合离 子,水解体系非常复杂。当水解pH在2以下,溶液中OH-浓度小于10-12 mol/L,可以忽略Sb3+在溶液中与OH-的配位[13]。盐酸浸取液中Cl-浓度较高,与Sb3+络合能力较强,易与SbO+按摩尔比1:1结合形成固体SbOCl,其水解反应如下[14]:
SbCl3+H2O= SbOCl↓+2HCl (3)
在稀释后液中加入氢氧化钠溶液,调节稀释液pH,使铋形成沉淀。当温度为20 ℃,反应时间为1 h时,终点pH对铋水解率的影响如图3所示,水解产物XRD图和成分分别如图4和表2所示。
由图3可知:随着终点pH的增加,铋水解率增大。当pH为1.3时,铋的水解率为80%,当pH≥1.5时,铋水解率大于99.5%。其水解反应为[15]:
BiCl3+H2O=BiOCl↓+2HCl (4)

图3 终点pH对铋水解率的影响
Fig.3 Effect of final pH on hydrolysis rate of Bi
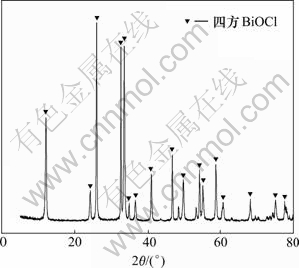
图4 铋水解产物XRD谱
Fig.4 XRD pattern of bismuth product
表2 铋水解产物成分(质量分数)
Table 2 Composition of bismuth product %

由图4可知:铋水解产物为氯氧铋。由表2可知:铋水解产物主要成分为铋、氧、氯,其质量分数分别为54.23%,19.30%和14.61%。
2.3 氯氧铋的浸出
氯氧铋中铋、锑、铅主要存在形态分别是BiOCl,SbOCl和PbCl2,砷主要以难溶的砷酸盐形式存在。采用高浓度盐酸浸取氯氧铋浸出,通过优化实验,当盐酸浓度为5 mol/L,反应温度为30 ℃,液固比为3:1,反应时间为1 h时,浸出结果如表3所示,所得浸出渣成分如表4所示。
表3 粗氯氧铋酸浸出率
Table 3 Hydrochloric acid leaching results of crude BiOCl %

表4 粗氯氧铋酸浸渣成分(质量分数)
Table 4 Composition of residue from crude BiOCl hydrochloric acid leaching %

实验结果表明氯氧铋经过盐酸浸出,锑、砷、铅和铁等杂质大部分留于渣中,部分进入盐酸浸出液。
2.4 三氧化二铋的制备
为了进一步除去杂质,对氯氧铋盐酸浸出液进一步稀释水解,实验取1 L氯氧铋浸出液,在稀释比为5:1时,水解后过滤所得滤渣成分如表5所示,滤液成分如表6所示。
取1 L水解除杂溶液,用1 mol/L的氢氧化钠调节溶液的pH为1.5,反应0.5 h后过滤,所得氯氧铋成分如表7所示。
表5 氯氧铋浸出液水解渣成分(质量分数)
Table 5 Composition of hydrolysis residue %

表6 水解液成分(质量分数)
Table 6 Composition of hydrolysis solution %

水解所得氯氧铋比表面大,吸附性强,氯氧铋中杂质主要由于吸附而产生。实验选择了硫酸洗涤。硫酸洗涤后,氯氧铋杂质成分如表8所示。
表7 氯氧铋成分(质量分数)
Table 7 Composition of BiOCl %

表8 硫酸洗涤后氯氧铋杂质成分(质量分数)
Table 8 Composition of BiOCl after washing with sulfuric acid %

由表7和表8比较可知:经过硫酸洗涤氯氧铋中杂质得到进一步除去。除杂后氯氧铋制备氧化铋采用氢氧化钠转化法,其反应原理如下:
2BiOCl+2NaOH=Bi2O3+2NaCl+H2O (5)
当氢氧化钠浓度为6 mol/L,液固比为3:1,反应温度为80 ℃时,反应2 h后过滤,用0.5 mol/L盐酸洗涤,烘干后产物成分如表9所示,产物XRD实验结果如图5所示,产物SEM像如图6所示。
由表9可知:实验所得氧化铋中铋含量为89.53%,氧化铋纯度达到99.81%。由图5可知:氧化铋的晶型为单斜的α-Bi2O3。由图6可知:氧化铋表面形貌为纤维状。
表9 产物氧化铋成分(质量分数)
Table 9 Composition of Bi2O3 product %


图5 产物氧化铋XRD谱
Fig.5 XRD pattern of Bi2O3 product

图6 产物氧化铋SEM像
Fig.6 SEM image of Bi2O3 product
上述氧化铋制备过程中砷进入溶液被除去,含砷废水可采用硫酸铜沉淀与二氧化硫还原以三氧化砷得到回收[16]。
3 结论
(1) 采用盐酸浸出Sb和Bi质量分数分别为32.67%和9.48%的漂浮阳极泥,当盐酸浓度为6 mol/L,固液比为1:5,反应温度为80 ℃,反应时间为1 h时,锑和铋浸出率分别达到99.17%和99.08%。
(2) 采用稀释法分离盐酸浸出液中锑铋,当稀释比为8:1时,锑水解率为98.13%,铋水解率为8.8%,稀释后水解产物中锑、氯、氧和砷的质量分数分别为50.00%,19.02%,14.50%和13.16%,铋的质量分数仅为0.16%。
(3) 在稀释后液中加入氢氧化钠溶液,当pH为1.5时,铋水解率达到99.5%,水解产物为氯氧铋,铋、氧、氯的质量分数分别为54.23%,19.30%和14.61%。氯氧铋经过盐酸浸出,稀释水解,氢氧化钠中和得到氯氧铋,其铋、氧、氯的质量分数分别为66.47%,19.90%和14.12%。
(4) 氯氧铋经过硫酸洗涤除杂后,采用氢氧化钠转化,当氢氧化钠浓度为6 mol/L,液固比为3:1,反应温度为80 ℃时,反应2 h后过滤,用0.5 mol/L盐酸洗涤,烘干得到形貌为纤维状,晶型为单斜的α-Bi2O3,铋的质量分数为89.53%,氧化铋纯度达到99.81%。
参考文献:
[1] ZHENG Ya-jie, XIAO Fa-xin. A novel technology of purification of copper electrolyte[J]. Journal of China Nonferrous Metal, 2007, 17(5): 1069-1074.
[2] 彭容秋. 铜冶金[M]. 长沙: 中南大学出版社, 2004: 226-229.
PENG Rong-qiu. Copper metallurgy[M]. Changsha: Central South University Press, 2004: 226-229.
[3] 卢宜源. 贵金属冶金学[M]. 长沙: 中南大学出版社, 2004: 60-100.
LU Yi-yuan. Metallurgy of precious metals[M]. Changsha: Central South University Press, 2004: 60-100.
[4] 邱光文, 徐远志. 高银铜阳极泥湿法处理流程研究[J]. 有色金属设计, 2000, 27(2): 19-24.
QIU Guang-yuan, XU Yuan-zhi. Research of wet process of high content of silver copper anode slime[J]. Nonferrous Metals Design, 2000, 27(2): 19-24.
[5] 郑雅杰, 孙召明, 汪蓓, 等. 阳极泥预处理及回收稀散金属的方法: 中国, 200810032000.0[P]. 2009-01-07.
ZHENG Ya-jie, SUN Zhao-ming, WANG Bei, et al. Method of pretreatment of anode slime and recovery of rare metal: China, 200810032000.0[P]. 2009-01-07.
[6] 郑雅杰, 王蓓. 铜阳极泥预处理新工艺研究[J]. 中南大学学报: 自然科学版, 2010, 41(3): 865-870.
ZHENG Ya-jie, WANG Bei. Study on a novel pretreatment process for high lead copper anode slime[J]. Journal of Central South University: Science and Technology, 2010, 41(3): 865-870.
[7] Fernández M A, Segarra M, Espiell F. Selective leaching of arsenic and antimony contained in the anode slimes from copper refining[J]. Hydrometallurgy, 1996, 41(23): 255-267.
[8] 蔡练兵, 刘维, 柴立元. 高砷铅阳极泥预脱砷研究[J]. 矿冶工程, 2007, 12(6): 44-47.
CAI Lian-bing, LIU Wei, CHAI Li-yuan. Study on pre-treatment process of arsenic removal for arsenic-rich lead anode slime[J]. Mining and Metallurgical Engineering, 2007, 12(6): 44-47.
[9] 郑雅杰, 洪波. 漂浮阳极泥富集金银及回收锑铋工艺[J]. 中南大学学报: 自然科学版, 2011, 42(8): 2221-2226.
ZHENG Ya-jie, HONG Bo. Enrichment of Au and Ag and recovery of Sb and Bi from floating anode slime[J]. Journal of Central South University: Science and Technology, 2011, 42(8): 2221-2226.
[10] 陈进中, 曹华珍, 郑国渠, 等. 高锑低银类铅阳极泥制备五氯化锑新工艺[J]. 中国有色金属学报, 2008, 18(11): 2094-2099.
CHEN Jin-zhong, CAO Hua-zhen, ZHENG Guo-qu, et al. Novel technology for preparation of SbCl5 from lead anode slime with high antimony and low silver content[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(11): 2094-2099.
[11] 赵瑞荣, 石西昌. 冶金物理化学[M]. 长沙: 中南大学出版社, 2006: 35-151.
ZHAO Rui-rong, SHI Xi-chang. Metallurgical physical chemistry[M]. Changsha: Central South University Press, 2006: 35-151.
[12] 周磊, 李军旗. 一种用氧化锑矿、硫氧混合锑矿生产三氧化二锑粉的方法: 中国, CN1103895[P]. 1995-06-21.
ZHOU Lei, LI Jun-qi. A method of produce antimony trioxide powder usage antimony oxide ore and antimony sulfur-oxygen mixture ore: China, CN1103895[P]. 1995-06-21.
[13] 郑国渠, 支波, 陈进中. 五氯化锑的水解过程[J]. 中国有色金属学报, 2006, 16(9): 1628-1633.
ZHENG Guo-qu, ZHI Bo, CHEN Jin-zhong. Hydrolysis of antimony pentachloride[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(9): 1628-1633.
[14] 王庆祥, 郑蒂基, 傅崇说. 关于氯化物溶液中铅、银结晶分离的理论分析[J]. 中南矿冶学院学报, 1983, 36(2): 121-128.
WANG Qing-xiang, ZHENG Di-ji, FU Chong-shuo. A theoretical analysis on the crystallization separation of lead and silver in chloride solution[J]. Journal of Central South Institute of Mining and Metallurgy, 1983, 36(2): 121-128.
[15] 王云燕, 彭文杰, 舒余德, 等. Bi(Ⅲ)-X(Cl-, NO3-)-H2O体系热力学平衡研究[J]. 中南工业大学学报: 自然科学版, 2001, 32(3): 139-141.
WANG Yun-yan, PENG Wen-jie, SHU Yu-de, et al. Thermodynamic equilibrium study of Bi(Ⅲ)-X(Cl-, NO3-)-H2O system[J]. Journal of Central South University of Technology: Natural Science, 2001, 32(3): 139-141.
[16] 郑雅杰, 罗园, 王勇. 采用含砷废水沉淀还原法制备三氧化二砷[J]. 中南大学学报: 自然科学版, 2009, 40(1): 48-54.
ZHENG Ya-jie, LUO Yuan, WANG Yong. Arsenic trioxide made by precipitation-reduction method from As-containing waste water[J]. Journal of Central South University: Science and Technology, 2009, 40(1): 48-54.
(编辑 杨幼平)
收稿日期:2011-07-22;修回日期:2011-10-11
基金项目:广东省科技厅资助项目 (2011B0508000033)
通信作者:郑雅杰(1959-),男,湖南常德人,博士,教授,从事冶金、水污染控制、资源综合利用研究;电话:0731-88836285;E-mail: zzyyjj01@yahoo.com.cn