AZ31B镁合金表面改性微弧氧化膜的结构及耐蚀性能
来源期刊:中国有色金属学报(英文版)2016年第3期
论文作者:崔学军 杨瑞嵩 刘春海 余祖孝 林修洲
文章页码:814 - 821
关键词:镁合金;微弧氧化;等离子体电解氧化;疏水;十四烷酸;耐蚀
Key words:magnesium alloys; micro-arc oxidation; plasma electrolytic oxidation; hydrophobicity; myristic acid; corrosion resistance
摘 要:利用十四烷酸溶液化学浸泡的方法改性微弧氧化AZ31B镁合金,获得了具有疏水特性的微弧氧化膜。采用接触角测量仪检测膜层的润湿性能;利用SEM、XRD和FT-IR等方法表征膜层的形貌和结构;通过极化曲线和浸泡实验考察样品在3.5% NaCl溶液中的耐蚀性能。结果表明:改性时间从0 h增至5 h时,膜层的静态接触角从0°增加到了138°,膜层的微孔尺寸减小,微孔数量减少;化学改性未改变微弧氧化膜的晶体结构,但改性样品的自腐蚀电位正移,自腐蚀电流密度降低,耐蚀性能得到提高,且疏水性和耐蚀性随着烷基链的增长而增强。氧化膜表面微观多孔的结构促进低表面能烷基羧酸的润湿铺展,烷基链通过双配位键合作用形成单分子层,封闭或缩小微孔,致密微弧氧化膜,并赋予其疏水特性,进一步提高微弧氧化膜对镁合金的腐蚀防护能力。
Abstract: A hydrophobic surface was fabricated on a micro-arc oxidation (MAO) treated AZ31 Mg alloys via surface modification with myristic acid. The effects of modification time on the wettability of the coatings were investigated using the contact angle measuring device. The surface morphologies and structure of the coatings were evaluated using SEM, XRD and FT-IR. The corrosion resistance was investigated by potentiodynamic polarization curves and long-term immersion test. The results showed that the water contact angle (CA) increases gradually with modification time from 0 to 5 h, the highest CA reaches 138° after being modified for 5 h, and the number and size of the micro pores are decreased. The modification method hardly alters crystalline structure of the MAO coating, but improves the corrosion resistance based on the much positive potential and low current density. Moreover, the corrosion resistance and hydrophobicity can be enhanced with increasing the alkyl chain. The wetting and spreading for the alkylcarboxylate with low surface energy become easier on the micro-porous surface, and alkylcarboxylate monolayer will be formed through bidentate bonding, which changes the surface micropores to a sealing or semi-sealing structure and makes the MAO coating dense and hydrophobic. All the results demonstrate that the modification process improves the corrosion protection ability of the MAO coating on AZ31B Mg alloy.
Trans. Nonferrous Met. Soc. China 26(2016) 814-821
Xue-jun CUI1,2, Rui-song YANG1,2, Chun-hai LIU1,2, Zu-xiao YU1,2, Xiu-zhou LIN1,2
1. College of Materials Science and Engineering, Sichuan University of Science and Engineering, Zigong 643000, China;
2. Materials Corrosion and Protection Key Laboratory of Sichuan Province, Sichuan University of Science and Engineering, Zigong 643000, China;
Received 23 April 2015; accepted 16 July 2015
Abstract: A hydrophobic surface was fabricated on a micro-arc oxidation (MAO) treated AZ31 Mg alloys via surface modification with myristic acid. The effects of modification time on the wettability of the coatings were investigated using the contact angle measuring device. The surface morphologies and structure of the coatings were evaluated using SEM, XRD and FT-IR. The corrosion resistance was investigated by potentiodynamic polarization curves and long-term immersion test. The results showed that the water contact angle (CA) increases gradually with modification time from 0 to 5 h, the highest CA reaches 138° after being modified for 5 h, and the number and size of the micro pores are decreased. The modification method hardly alters crystalline structure of the MAO coating, but improves the corrosion resistance based on the much positive potential and low current density. Moreover, the corrosion resistance and hydrophobicity can be enhanced with increasing the alkyl chain. The wetting and spreading for the alkylcarboxylate with low surface energy become easier on the micro-porous surface, and alkylcarboxylate monolayer will be formed through bidentate bonding, which changes the surface micropores to a sealing or semi-sealing structure and makes the MAO coating dense and hydrophobic. All the results demonstrate that the modification process improves the corrosion protection ability of the MAO coating on AZ31B Mg alloy.
Key words: magnesium alloys; micro-arc oxidation; plasma electrolytic oxidation; hydrophobicity; myristic acid; corrosion resistance
1 Introduction
Nowadays, there has been an increasing attention focused on corrosion protection of Mg alloys because the poor corrosion resistance has severely restricted their widespread application in the automotive, aerospace and electronics industries [1-4]. An effective approach to protect Mg alloys from corrosion is to create a super-hydrophobic surface with a large water contact angle (>150°) and a sliding angle (<10°), which can exhibit better resistance to corrosion compared with a hydrophilic surface [5-13]. However, there are two inevitable problems when producing a super- hydrophobic surface on Mg alloys [7]. One is the high chemical and electrochemical activity. Mg alloys are susceptible to reacting with many substances, such as acid, alkali, and saline solutions; so, some methods have only been applied to the metals which are relatively inert or can form tight passivation films on their surface like copper, aluminium, and titanium [14-17]. Consequently, some approaches mentioned in literatures could be impracticable for use with Mg alloys due to the possible side reactions. Another problem is that the chemical activity is not homogeneous because Mg alloys consist of more than two phases. For the above reasons, a two-step process is involved in fabricating an artificial super-hydrophobic coating on Mg alloys: to form a rough micro/nanostructured surface, and then modify the surface using low surface energy materials. Using this principle, a super-hydrophobic layer was prepared on Mg alloys, and demonstrated to exhibit better corrosion resistance [7-13]. Although these methods are relatively simple to create super-hydrophobic surfaces on Mg alloys, some of them used relatively expensive reagents to prepare a micro/nanostructured surface, such as Ni [9,10], Co [11], and Ag [12]. Furthermore, fluorinated chemicals, which are expensive and may harm the human body and environment, are still used as hydrophobic modifying reagents [6,8]. Therefore, it is exigent to explore a green and more effective approach to retard the corrosion of Mg alloys.
Micro-arc oxidation (MAO), also known as plasma electrolytic oxidation (PEO) and anodic spark deposition, is a promising process to fabricate a high corrosion and wear resistant coating on Mg alloys due to its simplicity and low cost [18-25]. Meanwhile, the structure of an MAO coating on an Mg alloy typically consists of a porous outer-layer and a thin barrier layer [18-20]. The presence of a higher pore density on the surface of the MAO coating increases the effective surface area and thus the tendency for a corrosive medium to adsorb and concentrate into these pores. Subsequently, the corrosive medium could quickly infiltrate into the inner regions of the coating, contact and interact with the substrate, and consequently deteriorate the corrosion protection of the coating through localised changes in pH value [21,22]. Fortunately, the pores formed in the MAO coating can contribute to releasing the residual stress of the coating during micro-arc discharge [23], and produce a mechanical interlocking effect to improve the adhesion of topcoats [24,25]. In addition, the porous out-layer can also offer a rough surface to create hydrophobic coating, further to enhance the corrosion resistance of the MAO coating on Mg alloys [13,26,27].
Based on the two-step process to create an artificial super-hydrophobic coating on Mg alloys, we present a simple and efficient fabrication process of a hydrophobic surface on AZ31 Mg alloy. Wherein MAO technology was employed to first prepare an MAO coating with a rough micro-pore structure, which was then modified using a green and long chain substance with a low surface energy [13]. Recently, the researches on an MAO coating modified by myristic acid and stearic acid were carried out. The results show that the surface morphology and corrosion resistance of the modified MAO in the former are not as good as that of the coating in the later (stearic acid), resulting in that less research on myristic acid was performed. This work is focused on the structure and corrosion resistance of the modified MAO coating by myristic acid, obtaining a comparison of corrosion resistance between the myristic acid and stearic acid.
2 Experimental
2.1 Materials
All solvents and chemicals were purchased from Chengdu Kelong Chemical Reagent Co., China, and were of chemically pure grade, and used without further purification. The substrate material used for this investigation was an AZ31B Mg alloy with a chemical composition (mass fraction) of 3.1% Al, 0.9% Zn, 0.23% Mn, 0.1% Si, 0.01% Cu, 0.005% Ni, 0.003% Fe and balance Mg. All samples were cut to sizes of 30 mm × 20 mm × 2 mm.
2.2 Pre-treatment
First, the cut Mg alloy samples were degreased in an alkaline solution containing 45 g/L NaOH, 10 g/L Na3PO4·12H2O, and 0.1 g/L sodium dodecyl sulfate (C12H25ONaSO3) at 65 °C for 10 min, and then rinsed with deionized water. Second, the samples were metallographically ground using 600-1200 grit silicon carbide paper, rinsed with deionized water, ultrasonically degreased in acetone for 30 min, and then dried in warm air.
2.3 MAO coating
The AZ31B Mg alloy samples were used as the anode, and a stainless steel cylinder with 150 mm in diameter and 300 mm in length was used as the cathode during the MAO process. The electrolyte was made by dissolving 15 g/L Na2SiO3, 3.0 g/L NaF, 20 g/L NaOH, and 1.0 mL/L glycerol in deionized water, and then placed into the cylinder. The stainless steel cylinder was placed in a water-cooled pipe to keep the temperature of the solution below 45 °C during the MAO treatment. The preparation of the MAO coating was carried out with a fixed voltage using a 30 kW AC power supply during the MAO process. The voltage, frequency, duty cycle, and duration time were 260 V, 300 Hz, 30%, and 10 min, respectively. Subsequently, the MAO-treated samples were rinsed for 10 min in an ultrasonic bath, and then dried in warm air.
2.4 Modification
The cleaned MAO-treated specimens were immersed into an ethanol solution of 0.2 mol/L myristic acid (CH3(CH2)12COOH) for 0 h (MAO AZ31+0 h), 1 h (MAO AZ31+1 h), 3 h (MAO AZ31+3 h), or 5 h (MAO AZ31+5 h), and then were placed into drying oven to dry at 60 °C for 1 h.
2.5 Characterization and testing
Water contact angle was measured by an optical contact angle-measuring device (JC2000D, POWEREACH, China) at ambient temperature with a water droplet of about 5 μL. An average contact angle value was determined by measuring five different spots for each sample. The surface morphologies of the coatings were investigated by SEM (VEGA 3 SBU, Tescan, Czech Republic). The XRD (D2 PHASER, Bruker, Germany) operated with Cu Kα radiation was used to determine the phase composition of the coatings. Infrared spectra of the modified MAO coating were recorded with a FT-IR spectrometer (NICOLET 6700, Thermo Scientific, USA) using KBr pellets at ambient temperature.
All electrochemical experiments were performed in a 3.5% NaCl aqueous solution at room temperature using an electrochemical system (CHI660E, Shanghai Chenhua, China). An electrochemical cell with a three-electrode configuration was used, consisting of a saturated calomel electrode (SCE) as reference electrode, a platinum sheet as auxiliary electrode, and the coated samples as working electrodes. The working electrode was set in a custom- made Teflon holder, which had a circular window of 1 cm2 exposed to the electrolyte solution. The open circuit potentials (OCPs) of the samples were recorded after immersion in the 3.5% NaCl solution for ~30 min for their stabilization at room temperature (25 °C). Then, the potentiodynamic polarization (PDP) curves of the samples were recorded using a scanning rate of 1.0 mV/s and a scanning region set to ±500 mV with respect to the OCP. The electrochemical parameters such as corrosion potential (φcorr), corrosion current density (Jcorr), and cathodic Tafel slopes (bc) were derived from the cathodic polarization curves by Tafel extrapolation using an electrochemical software (CHI, Version 12.23, USA). The Tafel slop potential range was -60 mV with respect to Jcorr. These tests were repeated at least three times for reliability and reproducibility.
In order to further prove the long-term corrosion resistance of the coatings, a total immersion test was investigated in a 3.5% NaCl solution. The ratio of solution volume to a sample surface’s area is 20 mL/cm2 according to ASTM G31-72 (2004). After the immersion tests, the corrosion products were removed in a chromic acid solution (200 g/L CrO3+10 g/L AgNO3).

Fig. 1 Surface morphologies and contact angles (insets) of MAO coatings modified by myristic acid for 0 h (a), 1 h (b), 3 h (c) and 5 h (d), and stearic acid for 10 h (e) and high magnification view (f) of Fig. 1(e)
3 Results and discussion
3.1 Microstructure and wettability
Figure 1 shows the surface morphologies and CAs of the MAO coatings modified for different time. It is obvious from this that some pores, island-structures, and volcano-like structures are visible in Fig. 1(a), which is generally considered typical features of MAO coating [18-20]. With increasing the modification time, some sealing or semi-sealing structures of the surface pores are observed (Figs. 1(b)-(d)). These results indicate that the surface structure of the MAO coating is changed by the modification of myristic acid. Nevertheless, the surface of the modified coating by the stearic acid is more uniform and smooth than that of the modified coating by the myristic acid (Figs. 1(d)-(f)) [13]. This result should be ascribed to the higher acidity of the myristic acid than the stearic acid, leading to an uneven corroded surface.
The water droplet on the surface of the unmodified MAO coating spreads out over the surface, and the static contact angle is nearly 0° (inset in Fig. 1(a)), which allows the water droplet to easily wet and to quickly penetrate into the coating. In contrast, the water droplets show a very spherical shape on the surface of the modified MAO coatings, indicating that the surfaces of the modified MAO coatings exhibit distinctly hydrophobic properties. Additionally, the contact angle increases with increasing modification time (Figs. 1(b)-(d)), and a maximum contact angle of 138° is reached after modification for 5 h, implying that the CH3(CH2)12COO— was successfully adsorbed on the micro-arc oxidation AZ31 Mg alloy.
Comparing the static CAs in Figs. 1(c) and (d), it is obvious that the value increases less when the immersed time was increased from 3 to 5 h. In experiment, even though the immersed time would be 10 h or longer, the values of the angles are still between 130° and 140°, which reach a nearly constant value. The value of a static CA increases with increasing hydrocarbon chain length, indicating that higher hydrophobicity can be provided via the carboxylate ion with longer alkyl tails [28]. A maximum static contact angle of 151.5 was obtained by the same method in an ethanol solution of stearic acid [13], suggesting that the hydrophobic nature is not only related to the surface structure of the substrate, but also related to the substance of the adsorbed layer.
In order to conveniently analyse and discuss the related results, the MAO coating modified for 3 h is called the H-MAO coating (i.e., hydrophobic MAO coating) from this point forward.
3.2 XRD patterns
The XRD patterns obtained from an MAO coating and an H-MAO coating are shown in Fig. 2. It is clear that the MAO coating consists of MgO, MgSiO3 and MgF2, which indicates that both the Mg substrate and electrolyte were involved in its formation. The Mg peaks imply that the X-rays are capable of penetrating the coating to reach the substrate. Although there is a slight difference for their position between the MAO coating and H-MAO coating, no new phases appear in the pattern of the H-MAO coating. It is noted that the diffraction peak intensity of MgO is slightly decreased, and other peaks, e.g., MgSiO3 and Mg, are remarkably increased. This suggests that MgO and amorphous phases in the MAO coating, or more specifically its outer layer, may be dissolved during the modification process [13]. This is also concordant with the formation of precipitates that were observed during the modification of the MAO-treated samples.
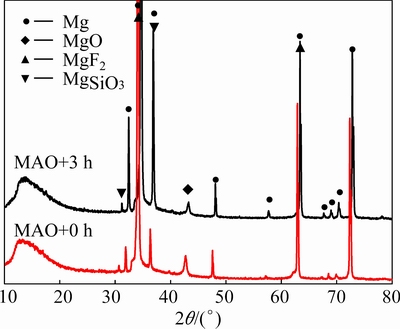
Fig. 2 XRD patterns of MAO coating and H-MAO coating
3.3 FT-IR characterization
In order to prove the existence of CH3(CH2)12COO— and its bonding to the MAO coating, FT-IR characterization was employed. As shown in Fig. 3, the high-frequency region of the spectra obtained displays the adsorption peaks at about 2925 and 2855 cm-1, which are attributed to C—H asymmetric and symmetric stretching vibrations, respectively [5,29]. In the low frequency region, the peak for the carboxyl group (—COO) from myristic acid should appear at 1701 or 1702 cm-1 [5,29,30], but this peak is no longer present on the H-MAO coating. This indicates that an interaction between the carboxylic acid head group and the MAO surface could occur, proving the bonding of CH3(CH2)12COO— on its surface through forming a bidentate mode on the surface [31]. Meanwhile, the two adsorption peaks at 1464 and 1424 cm-1, are owing to the carboxyl group symmetric stretching vibrations. No asymmetric stretching vibration peaks of carboxyl group are found between 1540 and 1560 cm-1, which implies that two carboxylate oxygen atoms are bonded to the surface with a symmetric bonding mode [5,29].
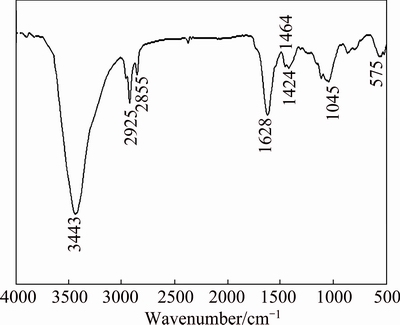
Fig. 3 FT-IR spectrum of H-MAO coating
3.4 PDP curves
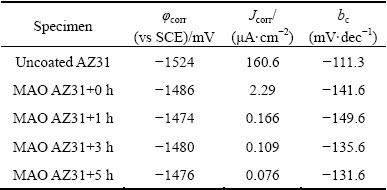
Figure 4 depicts PDP curves of an uncoated AZ31 Mg alloy and MAO coatings modified for different time in a 3.5% NaCl solution. The relevant fitting results, i.e., φcorr and Jcorr, are illustrated in Table 1. The φcorr and Jcorr values of the uncoated AZ31 Mg alloy were determined to be -1524 mV (vs SCE) and 160.6 μA/cm2, respectively, which are in good accordance with the results in the literature [32]. The MAO-coated AZ31 Mg alloy reveals a much lower Jcorr (2.29 μA/cm2), which represents a decrease of two orders of magnitude when compared with the uncoated substrate. Moreover, the φcorr of the MAO-treated sample slightly shifts to a more positive potential (about 40 mV), which should be caused by the change in the balance of anodic and cathodic reactions on the surface that are indirectly affected by the porous surface [33]. These results indicate that the MAO coating has a low corrosion rate and provides good corrosion resistance for AZ31 Mg alloy. Similarly, it is evident that the modified MAO coatings exhibit an even more positive φcorr (between 6 and 12 mV) and a lower Jcorr (nearly two orders of magnitude) compared with the MAO-treated sample, which indicates that the corrosion resistance of MAO coating is significantly enhanced by the hydrophobic modification.
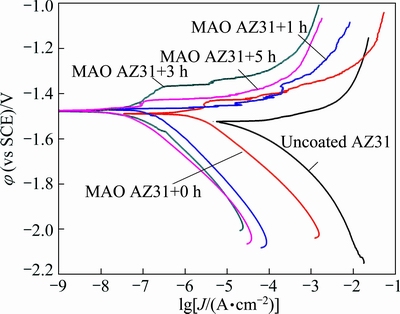
Fig. 4 PDP curves of AZ31 Mg alloy in uncoated state, and with MAO coatings modified for different time in 3.5% NaCl solution
According to Fig. 4 and Table 1, the bc values of the MAO-treated samples are almost similar before and after modification, indicating that the cathodic reaction is influenced slightly by the MAO coating and hydrophobic modification. While, the anodic polarization curves of the modified MAO coatings show evidence of a passivation behaviour, which demonstrated that the anodic reactions can be further inhibited. Therefore, the Jcorr values were determined from the cathodic polarization curves using Tafel extrapolation. Compared with the MAO-treated AZ31 Mg alloy, the modified specimens show a sharp anodic polarisation curve and an increasing passivation zone (modified for 3 h or 5 h in Fig. 4), as well as a more positive φcorr and a lower Jcorr (Table 1). These results further prove the enhanced corrosion resistance of modified MAO coating.
Table 1 Fitting results of PDP curves related to Fig. 4
3.5 Long-term immersion test
In order to further investigate the long-term corrosion resistance of the coatings, a total immersion test was performed in a 3.5% NaCl solution. The photographs of various samples after immersion in the 3.5% NaCl solution for 10 d are shown in Fig. 5. As shown in Figs. 5(a)-(c), the surface of uncoated AZ31 Mg alloy is completely covered by corrosion products, and the surface of MAO coating has some corrosion pits, which is in good agreement with the results of electrochemical analysis. Meanwhile, the H-MAO coating shows very good corrosion resistance, with few substances present on its surface in Fig. 5(c).
To clearly observe the surface of the corroded samples, they were treated to remove any corrosion products, as shown in Figs. 5(d)-(f). The uncoated substrate suffers complete corrosion damage after 10 d of immersion (Fig. 5(d)). Actually, the uncoated AZ31 Mg alloy is corroded completely after 3 d of immersion in a 3.5% NaCl solution [13]. Whereas, the MAO coating undergoes some severe localized corrosion on its surface (Fig. 5(e)), indicating that the MAO coating reduces the corrosion of the AZ31 Mg alloy. Meanwhile, the H-MAO coating only shows slightly localized areas of corrosion (mostly at its margins) after 10 d of immersion (Fig. 5(f)), clearly demonstrating that an H-MAO coating offers better long-term corrosion protection for AZ31 Mg alloy.

Fig. 5 Photographs of samples after immersion in 3.5% NaCl solution for 10 d with and without clearing of any corrosion products
When an uncoated AZ31 Mg alloy or an MAO- treated sample is immersed in a NaCl solution, Cl- can directly interact with the substrate, or interact with the outer porous layer of the MAO coating, and may even interact with the substrate through its outer porous layer, resulting in corrosion [7]. Conversely, a hydrophobic layer that was formed on the MAO coating, is capable of trapping a large amount of air, and so prevents the NaCl solution from directly contacting with the surface of the coating [7,9]. Thus, the air that is trapped in the pores of the surface retards the interaction between the Cl- and the substrate, which explains why the H-MAO coating exhibits the better corrosion resistance than the MAO coating and the uncoated substrate in a 3.5% NaCl solution.
The rough surface with micro pores on the AZ31 Mg alloy can be provided by micro-arc oxidation treatment, which is capable of trapping a large amount of air. Therefore, the Cassie equation [34] was used to explain the hydrophobicity of the surface.
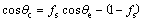 (1)
(1)
where θc is the contact angle on the rough surface, θe is the contact angle on a smooth surface with the same chemical nature, fs is defined as the fraction of the solid-liquid contact area, and 1-fs is the air fraction below the droplets. The equilibrium contact angles [28] are 104° and 105° for C11H21COONa and C15H29COONa on a smooth AZ31 Mg alloy surface, respectively. Therefore, the contrast contact angle on a smooth surface for myristic acid can be about 104.5°. From Eq. (1), the estimated fs is about 0.34 when the θc is 138° (Fig. 1(d)), indicating that the fraction of the air trapped within the interstices of the surface is approximately 0.66. This value is larger than that modified by stearic acid on AZ31 Mg alloy [13], implying the poor corrosion resistance.
4 Conclusions
1) A hydrophobic surface on an MAO-treated Mg alloy was developed via surface modification using myristic acid. The modification method cannot alter crystalline structure of the MAO coating, but changes the surface micro pores to a sealing or semi-sealing structure.
2) The static contact angle increases gradually with modification time from 0 to 5 h, and the maximum contact angle reaches 138° after being modified for 5 h. Compared with the MAO coating, the modified MAO coating shows higher potential, lower corrosion current density, and enhancing corrosion resistance. It is noted that the surface of the modified coating by stearic acid is more uniform and smooth than that of the modified coating by myristic acid, and exhibits better corrosion resistance.
3) The myristic acid penetrates into the porous outer layer of the MAO coating, and attaches to the MAO coating through bidentate bonding, namely, two carboxylate oxygen atoms symmetrically bond with the surface. This is responsible for the excellent water-repellent property and good corrosion protection ability.
Acknowledgements
The authors would like to thank Ying WANG for her assistance with the experiments.
References
[1] SONG G L. Recent progress in corrosion and protection of magnesium alloys [J]. Adv Eng Mater, 2005, 7: 563-586.
[2] LIU Wei, XU Dong-duo, DUAN Xiao-yue, ZHAO Guo-sheng, CHANG Li-min, LI Xin. Structure and effects of electroless Ni-Sn-P transition layer during acid electroless plating on magnesium alloys [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(5): 1506-1516.
[3] ZENG Rong-chang, LIU Zhen-guo, ZHANG Fen, LI Shou-qi, HE Qing-kun, CUI Hong-zhi, HAN En-hou. Corrosion resistance of in-situ Mg-Al hydrotalcite conversion film on AZ31 magnesium alloy by one-step formation [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(6): 1917-1925.
[4] CUI X J, LIU C H, YANG R S, FU Q S, LIN X Z, GONG M. Duplex-layered manganese phosphate conversion coating on AZ31 Mg alloy and its initial formation mechanism [J]. Corros Sci, 2013, 76: 474-485.
[5] ZHAO L, LIU Q, GAO R, WANG J, YANG W L, LIU L H. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance [J]. Corros Sci, 2014, 80: 177-183.
[6] ISHIZAKI T, SAITO N. Rapid formation of a superhydrophobic surface on a magnesium alloy coated with a cerium oxide film by a simple immersion process at room temperature and its chemical stability [J]. Langmuir, 2010, 26: 9749-9755.
[7] WANG Z W, LI Q, SHE Z X, CHEN F N, LI L Q, ZHANG X X, ZHANG P. Facile and fast fabrication of superhydrophobic surface on magnesium alloy [J]. Appl Surf Sci, 2013, 271: 182-192.
[8] GAO R, LIU Q, WANG J, ZHANG X F, YANG W L, LIU J Y, LIU L H. Fabrication of fibrous szaibelyite with hierarchical structure superhydrophobic coating on AZ31 magnesium alloy for corrosion protection [J]. Chem Eng J, 2014, 241: 352-359.
[9] LIU Y, YIN X M, ZHANG J J, YU S R, HAN Z W, REN L Q. An electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy [J]. Electrochi Acta, 2014, 125: 395-403.
[10] SHE Z X, LI Q, WANG Z W, LI L Q, CHEN F N, ZHOU J C. Researching the fabrication of anticorrosion superhydrophobic surface on magnesium alloy and its mechanical stability and durability [J]. Chem Eng J, 2013, 228: 415-424.
[11] CHU Q W, LIANG J, HAO J C. Facile fabrication of a robust super-hydrophobic surface on magnesium alloy [J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2014, 443: 118-122.
[12] JIA J, FAN J F, XU B S, DONG H B. Microstructure and properties of the super-hydrophobic films fabricated on magnesium alloys [J]. J Alloy Compd, 2013, 554: 142-146.
[13] CUI X J, LIN X Z, LIU C H, YANG R S, ZHENG X W, GONG M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy [J]. Corros Sci, 2015, 90: 402-412.
[14] WANG P, ZHANG D, QIU R, WAN Y, WU J. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance [J]. Corros Sci, 2014, 80: 366-373.
[15] HUANG Y, SARKAR D K, GALLANT D, CHEN X G. Corrosion resistance properties of superhydrophobic copper surfaces fabricated by one-step electrochemical modification process [J]. Appl Surf Sci, 2013, 282: 689-694.
[16] QIAN B T, SHEN Z Q. Fabrication of super-hydrophobic surface by dislocation selective chemical etching on aluminum, copper, and zinc substrates [J]. Langmuir, 2005, 21: 9007-9009.
[17] GUO M, KANG Z, LI W, ZHANG J. A facile approach to fabricate a stable super-hydrophobic film with switchable water adhesion on titanium surface [J]. Surf Coat Technol, 2014, 239: 227-232.
[18] WANG Shu-yan, SI Nai-chao, XIA Yong-ping, LIU Li. Influence of nano-SiC on microstructure and property of MAO coating formed on AZ91D magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(6): 1926-1934.
[19] CHU Cheng-lin, HAN Xiao, BAI Jing, XUE Feng, CHU Paul-kao. Surface modification of biomedical magnesium alloy wires by micro-arc oxidation [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1058-1064.
[20] ZOU Bin,  Guo-hua, ZHANG Gu-ling, TIAN Yu-ye. Effect of current frequency on properties of coating formed by microarc oxidation on AZ91D magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(5): 1500-1505.
Guo-hua, ZHANG Gu-ling, TIAN Yu-ye. Effect of current frequency on properties of coating formed by microarc oxidation on AZ91D magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(5): 1500-1505.
[21] MALAYOGLU U, TEKIN K C, SHRESTHA S. Influence of post-treatment on the corrosion resistance of PEO coated AM50B and AM60B Mg alloys [J]. Surf Coat Technol, 2010, 205: 1793-1798.
[22] LIANG J, BALA SRINIVASAN P, BLAWERT C, DIETZEL W. Influence of pH on the deterioration of plasma electrolytic oxidation coated AM50 magnesium alloy in NaCl solutions [J]. Corros Sci, 2010, 52: 540-547.
[23] GU Y H, XIOANG W M, NING C Y, ZHANG J. Residual stresses in microarc oxidation ceramic coatings on biocompatible AZ31 magnesium alloys [J]. J Mater Eng Perform, 2012, 21: 1085-1090.
[24] ARRABAL R, MOTA J M, CRIADO A, PARDO A, MOHEDANO M, MATYKINA E. Assessment of duplex coating combining plasma electrolytic oxidation and polymer layer on AZ31 magnesium alloy [J]. Surf Coat Technol, 2012, 206: 4692-4703.
[25] TANG Y, ZHAO X, JIANG K, CHEN J, ZUO Y. The influences of duty cycle on the bonding strength of AZ31B magnesium alloy by microarc oxidation treatment [J]. Surf Coat Technol, 2010, 205: 1789-1792.
[26] WANG S H, GUO X W, XIE Y J, LIU L H, YANG H Y, ZHU R Y, GONG J, PENG L M, DING W J. Preparation of superhydrophobic silica film on Mg-Nd-Zn-Zr magnesium alloy with enhanced corrosion resistance by combining micro-arc oxidation and sol–gel method [J]. Surf Coat Technol, 2012, 213: 192-201.
[27] GNEDENKOV S V, EGORKIN V S, SINEBRYUKHOV S L, VYALIY I E, PASHININ A S,EMELYANENKO A M, BOINOVICH L B. Formation and electrochemical properties of the superhydrophobic nanocomposite coating on PEO pretreated Mg-Mn-Ce magnesium alloy [J]. Surf Coat Technol, 2013, 232: 240-246.
[28] LIU Y L, YU Z F, ZHOU S X, WU L M. Self-assembled monolayers on magnesium alloy surfaces from carboxylate ions [J]. Appl Surf Sci, 2006, 252: 3818-3827.
[29] LIU Q, KANG Z X. One-step electrodeposition process to fabricate superhydrophobic surface with improved anticorrosion property on magnesium alloy [J]. Mater Lett, 2014, 137: 210-213.
[30] WANG Y, WANG W, ZHONG L, WANG J, JIANG Q, GUO X. Super-hydrophobic surface on pure magnesium substrate by wet chemical method [J]. Appl Surf Sci, 2010, 256: 3837-3840.
[31] HAN M, GO S, AHN Y. Fabrication of super-hydrophobic surface on magnesium substrate by chemical etching [J]. Bull Korean Chem Soc, 2012, 33: 1363-1366.
[32] LIM T S, RYU H S, HONG S H. Electrochemical corrosion properties of CeO2-containing coatings on AZ31 magnesium alloys prepared by plasma electrolytic oxidation [J]. Corros Sci, 2012, 62: 104-111.
[33] YAGI S, SENGOKU A, KUBOTA K, MATSUBARA E. Surface modification of ACM522 magnesium alloy by plasma electrolytic oxidation in phosphate electrolyte [J]. Corros Sci, 2012, 57: 4-80.
[34] CASSIE A B D, BAXTER S. Wettability of porous surfaces [J]. Trans Faraday Soc, 1944, 40: 546-551.
崔学军1,2,杨瑞嵩1,2,刘春海1,2,余祖孝1,2,林修洲1,2
1. 四川理工学院 材料科学与工程学院,自贡 643000;
2. 四川理工学院 材料腐蚀与防护四川省重点实验室,自贡 643000
摘 要:利用十四烷酸溶液化学浸泡的方法改性微弧氧化AZ31B镁合金,获得了具有疏水特性的微弧氧化膜。采用接触角测量仪检测膜层的润湿性能;利用SEM、XRD和FT-IR等方法表征膜层的形貌和结构;通过极化曲线和浸泡实验考察样品在3.5% NaCl溶液中的耐蚀性能。结果表明:改性时间从0 h增至5 h时,膜层的静态接触角从0°增加到了138°,膜层的微孔尺寸减小,微孔数量减少;化学改性未改变微弧氧化膜的晶体结构,但改性样品的自腐蚀电位正移,自腐蚀电流密度降低,耐蚀性能得到提高,且疏水性和耐蚀性随着烷基链的增长而增强。氧化膜表面微观多孔的结构促进低表面能烷基羧酸的润湿铺展,烷基链通过双配位键合作用形成单分子层,封闭或缩小微孔,致密微弧氧化膜,并赋予其疏水特性,进一步提高微弧氧化膜对镁合金的腐蚀防护能力。
关键词:镁合金;微弧氧化;等离子体电解氧化;疏水;十四烷酸;耐蚀
(Edited by Yun-bin HE)
Foundation item: Project (2014RC18) supported by Talent Introduction Funds of the Sichuan University of Science and Engineering, China; Project (2013CL01) supported by the Opening Project of the Material Corrosion and Protection Key Laboratory of Sichuan Province, China; Project (2013X06) supported by the Science and Technology Planning Project of Zigong City, China
Corresponding author: Xue-jun CUI; Tel: +86-813-5505620; E-mail: cxj_2046@163.com
DOI: 10.1016/S1003-6326(16)64172-9

