添加LaCl3对电沉积Ni-S涂层结构和电化学性能的影响
袁铁锤,周科朝,李瑞迪
(中南大学 粉末冶金国家重点实验室,湖南 长沙,410083)
摘要:以硫脲为硫源、LaCl3为添加剂,采用改进的Watt浴体系在泡沫镍基体上电沉积制备Ni-S涂层电极,采用SEM观察涂层表面形貌,采用XRD分析涂层结构,电化学测试方法研究涂层的电化学行为。研究结果表明:La3+在电沉积过程中对晶粒生长起阻碍作用,能够促进沉积层的晶粒细化,同时增大了沉积层的比表面积。所制备的涂层在碱性介质下具有较高的析氢活性和耐腐蚀性能。当镀液中LaCl3质量浓度为3 g/L时,制备的Ni-S涂层析氢过电位降低约38 mV,电极反应活化能为35.522 kJ/mol,在长时间水电解实验中具有较高的电化学活性和稳 定性。
关键词:LaCl3;Ni-S涂层;电沉积;析氢性能
中图分类号:TQ153.2 文献标志码:A 文章编号:1672-7207(2011)08-2285-06
Effect of additive LaCl3 on structures and electrochemical properties of nickel sulphur alloy coatings
YUAN Tie-chui, ZHOU Ke-chao, LI Rui-di
(State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract: Nickel-sulphur coating electrodes were prepared on foam nickel substrates by electrodeposition method in a modified Watts using thiourea (TU) and LaCl3 as sulphur source and additive, respectively. Adsorption effect of LaCl3 on the electrochemical activities, grain sizes, textures and microstructure of Ni-S coatings were characterized by means of SEM, XRD and electrochemical methods. The results show that LaCl3 can inhibit growth of coatings. The Ni-S coatings obtained from bath containing LaCl3 have larger surface area and much smaller grain sizes than those from the bath without additive. Ni-S alloy coating as a cathode for alkaline water electrolysis exhibits higher hydrogen evolution activities and better corrosion resistant performance. The hydrogen evolution overpotential of Ni-S coatings deposited at 3 g/L LaCl3 is 38 mV lower than that of the bath without additive. In the meantime, the Ni-S coatings have lower activation energy, which is 35.522 kJ/mol, and high electrochemical stability during long-term electrolysis.
Key words: LaCl3; Ni-S alloy coating; electrodeposition; hydrogen-evolution activities
氢能源作为一种高效、洁净和理想的二次能源已受到了世界各国的广泛关注。电解水制氢是实现工业大规模开发和利用氢能的重要手段。但目前全部能耗大部分与析氢过电位有关,过高的析氢过电位严重抑制了这种电极材料的应用。早期电解水制氢的阴极材料主要以贵金属Pt和Pd及其合金为主,这类合金超电势很低,电催化效果好,但其价格昂贵,难以应用于工业生产中。目前工业上应用最多的碱性水电解制氢的电极是Raney-Ni电极,但该电极一直存在强度和电位稳定性的问题。其次是Ni-S型合金电极。这种合金对碱性水电解是一种很好的阴极材料。如挪威Norsk Hydro公司用电沉积的方法在C/Ni基体上制备出电化学活性非常高的Ni-S电极[1],在25%的KOH溶液、80 ℃、电解电流密度为100 mA/cm2时,析氢过电位在6月内始终维持在60~110 mV。Ni-S阴极可以由多种方法制得,通常在瓦特(Watt)浴中加入KSCN[2],NaSCN[3]和硫脲[4](TU)等作为硫源。大量实验研究表明,Ni-S电极在碱性介质中具有较强的析氢催化活性,是一种应用前景广阔的阴极材料[5-7]。但Ni-S电极在使用过程中存在活性层易脱落、析硫、稳定性差等问题。稀土离子是少数能够在电极界面进行特性吸附的阳离子[8],特性吸附使电极界面双电层的结构发生变化。目前,稀土添加剂在电镀工业中应用非常广泛,特别是用于提高镀液的分散能力,改善镀层结构。稀土元素在电沉积Ni-P合金工艺中,可以促进电沉积过程中阴极极化,促进非晶组织的形成[9];在电沉积Ni-Co合金中,可增强镀层的耐腐蚀性,提高析氢电催化活性[10];Han等[11]在铜基体上电沉积制备了Ni-S(La)合金,研究表明,加入LaCl3可提高电极的析氢性能,降低析氢反应活化能。然而,稀土元素在电极表面吸附作用机理尚不十分明确。由于稀土元素具有4f层未充满的电子结构和独特的化学亲和力,因此,有必要进一步研究电沉积过程中稀土离子对镀层形核生长的影响规律。本文作者通过在Watt浴中添加LaCl3,采用电沉积的方法在具有立体结构的泡沫镍基体上制备出Ni-S涂层电极,用作碱性水电解。在Watt浴中用硫脲作硫源,采用稳态极化曲线测量涂层析氢活性,用XRD和SEM分析涂层的形貌和结构,用EDX测量镍硫涂层中的硫含量。
1 实验
1.1 Ni-S合金涂层电极的制备
采用阳极是长度×宽为1.5 cm×1.5 cm的镍片,阴极是长度×宽为1 cm×1 cm的泡沫镍,所用镍片及泡沫镍的镍含量(质量分数)均大于99%。电镀液基本组成为Watt浴体系:硫酸镍60~160 g/L,硫脲10~120 g/L,硼酸10~60 g/L,氯化钠5~40 g/L,三氯化镧0.2~3.5 g/L,添加剂和表面活性剂适量。采用双阳极单阴极,在30~60 ℃,5~60 mA/cm2,pH为3~6条件下进行电沉积。沉积时间为20~100 min。沉积完毕后,样品用去离子水清洗干净后自然干燥。
1.2 电极表面形貌和结构
用扫描电镜(X650)对镀层表面形貌进行观察,用面能谱扫描分析镀层表面元素含量,用XRD(D/MAX- RB)分析镀层物相组成、晶粒尺寸、结晶状态。
1.3 电化学性能测试
用泡沫镍基体(经预处理除油、酸洗)做工作电极,电解液与上述电镀液配方相同,参比电极为饱和Hg2Cl2,铂丝为对电极,用CHI660b(USA)进行电沉积过程极化曲线和阻抗测试,扫描速率为0.1 V/s,交流电频率为1 kHz,振幅为5 mV。用CHI660b(USA)恒电位仪测量涂层的电化学性能,采用欧姆补偿功能自动校正仪测试溶液的电位降。电解液为30% KOH水溶液,在不同温度下测定其阴极极化曲线和室温下的阳极极化曲线。同时,用上述电化学工作站,采用电位跃迁法测量电极的微分电容,从而求得电极真实表面积和相对粗糙度。
2 结果与讨论
2.1 稀土La对Ni-S电沉积过程的影响
图1所示为泡沫镍基体上Ni-S电沉积的阴极极化曲线。由图1可见:在Ni-S镀液中加入LaCl3后,电沉积过程的阴极极化增强。这是因为La3+具有较大的离子半径,当镀液中加入稀土La后,通过其特性吸附改变了电极界面双电层结构,阻碍了Ni2+,Ni[CS(NH2)2]2+和Ni[CS(NH2)2]22+络合离子的电沉积和界面扩散。从图1还可看出:在电位负向扫描初期,由于泡沫镍基体上无Ni-S成核中心,相应的电结晶生长未能发生,故电流接近于0 A。只有当电极电位达到成核所需的成核功时,电流才逐渐上升;负向扫描中阴极电流分别在-0.52 V(无稀土)和-0.8 V。(3 g/L LaCl3)以后才开始逐渐上升。说明LaCl3能阻碍镀层晶核的形成。
稀土La对Ni-S电沉积的影响可用阻抗-电位变化曲线加以验证。图2所示为泡沫镍基体上Ni-S电沉积的阻抗-电位关系。由图2可见:该体系阻抗-电位的变化关系与图1中沉积过程的极化曲线相对应。当Ni-S未沉积时,阻抗随电位变化较小,当电位达到能提供沉积Ni-S所需的成核功时,阻抗迅速下降,对应图1中的电流逐渐上升。若溶液中不存在LaCl3,电位负向扫描至-0.5 V附近,阻抗迅速下降,但过了-1.8 V之后,则随电位的负移而有所增大。当溶液中加入LaCl3后,上述阻抗迅速下降,其电位负移至-0.8 V附近,并在-1.95 V之后阻抗有所增大。造成上述变化的原因可能与阴极过程反应控制步骤的转变有关:在阻抗达到最小值之前,浓差极化还不显著,主要以电化学极化为主,此时电位越负,电化学反应阻抗就越小,随着阴极过电位的增加,浓差极化开始显著,最终成为控制步骤,此时,Warberg阻抗便不随阴极过电位增加而增加。由图2还可以看到:添加LaCl3后的阻抗大于无LaCl3时的阻抗。由于La3+在电极表面的吸附,产生了对Ni2+以及络合离子电还原沉积的阻碍作用,从而减缓了阴极和放电离子的交换速度,阻碍了晶粒的生长。

图1 泡沫镍基体上Ni-S沉积的阴极极化曲线
Fig.1 Cathode polarization curves of Ni-S coatings on foam nickel substrate

图2 泡沫镍基体上Ni-S沉积的阻抗-电位变化曲线
Fig.2 Change curves of impedance vs potential of Ni-S coatings on foam nickel substrate
2.2 稀土La对泡沫镍基Ni-S涂层形貌及结构的影响
图3所示为镀液中不同LaCl3质量浓度下Ni-S镀层的表面形貌。由图3可以看出:未加LaCl3的镀层表面较平坦,比表面积不够大;随着LaCl3质量浓度的不断增大,镀层表面变得粗糙,具有较大的表面。这是因为电沉积中控制步骤为电化学和扩散混合控制步骤。一方面,当镀液中加入LaCl3后,如果电极表面某些活性点被La3+大量吸附,那么该活性点附近的Ni2+及其络合离子的质量浓度大幅度减小,导致浓差极化,镀层生长被抑制,该处形成凹谷,而未被La3+大量吸附的区域镀层迅速生长形成尖峰;另一方面,随着电沉积反应的进行,由于扩散传质的原因,凹谷处由于浓差极化又被抑制生长,而尖峰处的电化学极化和浓差极化都比较低,故优先生长,形成了如图3所示的粗糙形貌。同时对上述电极进行EDX能谱分析,结果表明:加入LaCl3后对镀层的成分影响不明显,S质量分数均在18%左右,但没有发现La元素存在,因为稀土元素析出电位很负,很难从水溶液中直接沉积。
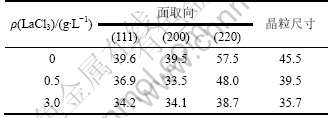
图4所示为不同LaCl3质量浓度下Ni-S电极的XRD图谱。由图4可见:Ni-S电极有很强的泡沫镍基体峰,但镍峰底部的XRD衍射峰出现一定宽化,这是因为Ni-S涂层为非晶态和纳米晶的混晶结构[12]。根据衍射线的宽化程度,利用Scherrer公式计算出电极的平均晶粒尺寸,计算结果如表1所示。从表1可以看出:Ni-S电极的平均晶粒尺寸随添加剂浓度的增大而有所减小。结果表明:La3+在电极表面的吸附导致超电势提高,阴极极化增大,阻碍了晶核生长,导致镀层晶粒尺寸减小。
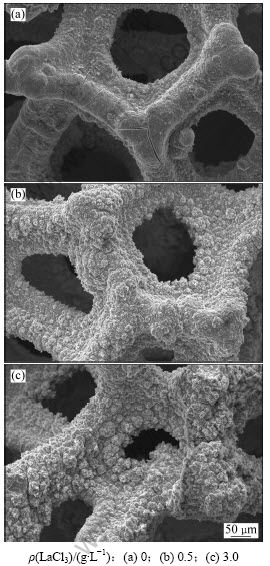
图3 不同LaCl3质量浓度下Ni-S镀层的SEM像
Fig.3 SEM images of Ni-S coatings with different LaCl3 mass concentrations

图4 不同LaCl3质量浓度下Ni-S涂层电极的XRD图谱
Fig.4 XRD patterns of Ni-S coating electrodes with different LaCl3 mass concentrations
表1 不同LaCl3质量浓度下Ni-S涂层电极的晶粒尺寸
Table 1 Grain size of Ni-S coating electrodes with different LaCl3 mass concentrations nm
从图4还可以看出:虽然XRD谱上均出现fcc镍结构的衍射峰,但各个晶面的衍射强度因子略有差异,即电极的择优取向发生变化。为考察涂层电极择优取向程度的变化,借鉴文献[13],采用下列公式计算晶面(hkl)表征晶面的择优取向指数TC:
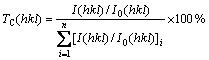 (1)
(1)
式中:TC(hkl)为计算的晶向择优取向指数;I(hkl)为实验测定的(hkl)面的XRD强度;I0(hkl)为JCPDS卡中的(hkl)面的XRD强度;n为衍射峰的个数,所取晶面分别是(111),(200)和(220)。由式(1)可知:TC(hkl)越大,表示其择优取向程度越高。
表2所示为不同LaCl3质量浓度下Ni-S涂层电极的择优取向系数。从表2可以看出:加入稀土后,电极(111)晶面的取向系数减少11%~15%,(200)晶面的取向因子增加约11%,说明镀液中加入LaCl3一方面可以吸附在电极表面抑制晶粒的生长,使晶粒细化;另一方面,能够使镀层(111)面生长速度变慢, (200)晶面的生长速度便快。有关稀土元素对镀层生长择优取向的影响还有待进一步研究。
表2 不同LaCl3质量浓度下Ni-S涂层电极的择优取向系数
Table 2 Texture coefficient of (hkl) planes of Ni-S coating electrodes with different LaCl3 mass concentrations %

2.3 稀土La对泡沫镍基Ni-S涂层电极析氢性能的 影响
图5所示为不同LaCl3质量浓度下涂层的阴极极化曲线。从图5可以看出:当LaCl3质量浓度达到3 g/L时涂层具有较好的析氢性能,在150 mA/cm2电流密度下电解时,该涂层比镀液中未加LaCl3的涂层析氢过电位降低约38 mV。这是由于析氢催化活性与电极表面粒子尺寸有直接关系,电极表面越粗糙,越有利于析氢。为此,用电位跃迁法测定了电极的微分电容Cd,假设平滑金属表面的平均微分电容为20 ?F/cm2,则电极的比表面积Sreal=Cd/20,电极的粗糙因子r=Sreal/Sapp。测量后计算所得的数据如表3所示。从表3可以看出:添加La后电极表面粗糙度变大,这与SEM和XRD分析结果一致。

图5 不同LaCl3质量浓度下Ni-S镀层的析氢极化曲线
Fig.5 Polarization curves of Ni-S coatings with Different LaCl3 mass concentrations
表3 电极的比表面积及相对粗糙因子
Table 3 Real areas and roughness factors of the electrodes

图6所示为Ni-S(LaCl3 3 g/L)电极在不同温度下过电位(η)随电流密度的变化曲线。表4所示为该电极在不同温度下的动力学参数。从表4可见:在碱性介质中温度对析氢电位的影响较明显;随着温度的升高,电极的交换电流密度J0随之增大,析氢电位明显降低。根据Arrhenius公式,电极析氢反应的真实交换电流密度与表观活化能及温度之间符合如下关系:
lgJ0=lg(FKc)-ΔG≠/(2.3(RT)) (2)
式中:F为法拉第常数;K为常数;c为反应物浓度;J0为电极交换电流密度;?G≠为表观反应活化能;R为气体常数。
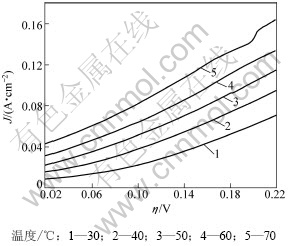
图6 不同温度下Ni-S(LaCl3 3 g/L)电极的η-J曲线
Fig.6 η-J curves of Ni-S(LaCl3 3 g/L) electrode at various temperatures
根据方程(2)做出lgJ0-1/T图,如图7所示。计算得到当镀液中LaCl3质量浓度为3 g/L时,沉积的Ni-S涂层电极表观活化能为35.522 kJ/mol。添加稀土La后Ni-S电极的表观反应活化能比未添加La电极的 低[12],说明添加La后涂层的析氢活性提高。
2.4 稀土La对Ni-S涂层电极耐腐蚀性能的影响
图8所示为Ni-S涂层在30% KOH溶液中的阳极极化曲线。从图8可以看出:镀液中加入LaCl3后得到的涂层阳极极化程度变大,即在相同的电极电位下,溶液中加入稀土La后得到的涂层具有较小的溶解电流和较长的钝化区,说明涂层的耐电化学腐蚀性增加。这可能是镀液中加入稀土La后镀层的晶粒变小,非晶态程度增加,而非晶态是一种长程无序短程有序的均质结构,表现出良好的耐腐蚀性,优于晶态镀层。
表4 LaCl3质量浓度为3 g/L时电极的析氢反应动力学参数
Table 4 Kinetics parameters of electrode with 3 g/L LaCl3 in plating liquid


图7 电极材料交换电流密度对数与T-1的关系
Fig.7 Relationship between exchange current density and T-1
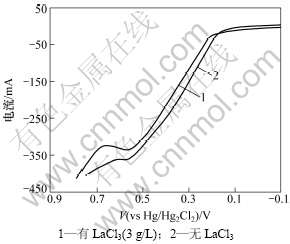
图8 Ni-S涂层的阳极极化曲线
Fig.8 Anodic polarization curves of Ni-S coatings with and without LaCl3(3 g/L)
2.5 Ni-S(La)电极稳定性研究
为了研究稀土LaCl3质量浓度为3 g/L时涂层电极的稳定性,将该电极在温度为60 ℃、质量分数为30% KOH溶液中,进行100 h连续电解(J=150 mA/cm2)实验。图9所示为析氢反应电位随时间变化关系。从图9可以看出:该电极经前100 h电解活化后,析氢电位基本稳定在-1.46~-1.50 V之间,表现出良好的电化学稳定性。

图9 Ni-S电极(LaCl3 3 g/L)的连续电解条件下电位随时间变化关系
Fig.9 Relationship between potential and time of Ni-S electrode (LaCl3 3 g/L) under long-term electrolysis
3 结论
(1) La3+通过在电极表面吸附,产生对Ni2+以及络合离子电还原沉积的阻碍作用,从而减缓了阴极和放电离子的交换速度,增大阴极极化,同时能够促进沉积层晶粒细化,增大沉积层的比表面积。
(2) 当镀液中LaCl3质量浓度为3 g/L时沉积的涂层具有较好的析氢性能,在电流密度150 mA/cm2下电解时,该涂层比未加LaCl3时涂层的析氢过电位降低约38 mV;通过对不同温度下析氢反应动力学参数分析,计算了该电极反应活化能为35.522 kJ/mol,说明添加稀土La有助于提高涂层的电化学活性。
(3) 当LaCl3质量浓度为3 g/L时,沉积的涂层表现出良好的耐腐蚀性能和电化学稳定性,电极经100 h连续电解,析氢电位基本稳定在-1.46~-1.50 V之间。
参考文献:
[1] Hydro N, Oslo. Electrolyte cell cathode with low overvoltage[P]. Netherlands Patent, 7801955. 1978.
[2] Hine F, Yasuda M, Watanabe M. Studies of the nickel-sulphur electrodeposited cathode[J]. Denki Kagaku, 1979, 47(4): 400-404.
[3] Gonsalez E. R, Avaca L. A. Tremiliosi-filho G. Hydrogen evolution reaction on Ni-S electrodes in alkaline solutions[J]. Inter J Hydrogen Energy, 1994, 19(1): 15-19.
[4] Paseka I. Sorption of hydrogen and kinetics of hydrogen evolution on amorphous Ni-Sx electrodes[J]. Electrochemica Acta, 1993, 38(16): 2449-2454.
[5] HAN Qing, LIU Kui-rong, CHEN Jian-she, et al. Hydrogen evolution reaction on amorphous Ni-S-Co alloy in alkaline medium[J]. Int J Hydrogen Energy, 2003, 28(1): 1345-1352.
[6] HAN Qing, LIU Kui-rong, CHEN Jian-she, et al. Study of amorphous Ni-S-Co alloy used as hydrogen evolution reaction cathode in alkaline medium[J]. Int J Hydrogen Energy, 2004, 29(3): 243-248.
[7] HE Han-wei, LIU Hong-jiang, LIU Fang, et al. Distribution of sulphur and electrochemical properties of nickel sulphur coatings electrodeposited on the nickel foam as hydrogen evolution reaction cathodes[J]. Materials Letters, 2005, 59(29/30): 3968-3972.
[8] 查全性. 电极过程动力学导论[M]. 北京: 科学出版社, 1987: 57-59.
ZHA Quan-xing. Introduction of electrode course kinetics[J]. Beijing: Science Press, 1987: 57-59.
[9] 邵光杰, 秦秀娟, 于升学, 等. 稀土对电沉积Ni-P合金镀层显微组织的影响[J]. 中国有色金属学报, 2000, 10(增1): 239-243.
SHAO Guang-jie, QIN Xiu-juan, YU Sheng-xue, et al. Influence of RE elements on microstructures of Ni-P alloy electro-plating coatings[J]. Transactions of Nonferrous Metals Society of China, 2000, 10(S1): 239-243.
[10] 朱龙章, 高玉香. 稀土(La,Nd,Sm)化合物对电沉积Ni-Co合金的影响[J]. 上海师范大学学报: 自然科学版, 2002, 31(4): 33-36.
ZHU Long-zhang, GAO Yu-xiang. The effects of rare earth (La, Nd, Sm) compounds on electrodeposition of Ni-Co alloy[J]. Journal of Shanghai Teachers University: Natural Science, 2002, 31(4): 33-36.
[11] HAN Qing, LIU Kui-ren, CHEN Jian-she, et al. Study of amorphous Ni-S(La) alloy used as HER cathode in alkaline medium[J]. Journal of Alloys and Compounds, 2005, 400(2): 265-269.
[12] YUAN Tie-chui, LI Rui-di, ZHOU Ke-chao. Electrocatalytic properties of Ni-S-Co coating electrode for hydrogen evolution in alkaline medium[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(4): 762-765.
[13] 黄琦晟, 钟云波, 任忠鸣, 等. 强磁场下铜电沉积层表面形貌及织构的研究[J]. 稀有金属材料与工程, 2006, 35(增2): 381-385.
HUANG Qi-sheng, ZHONG Yun-bo, REN Zhong-ming, et al. Surface crystal morphology and texture of electro-deposited layer of copper in static high magnetic field[J]. Rare Metal Materials and Engineering, 2006, 35(S2): 381-385.
(编辑 陈灿华)
收稿日期:2011-01-19;修回日期:2011-03-28
基金项目:国家高技术研究发展计划(“863”计划)项目(2003AA305980)
通信作者:袁铁锤(1968-),男,湖南新宁人,博士,副研究员,从事涂层及高纯金属材料的研究;电话:0731-88877186;E-mail:tiechuiyuan@csu.edu.cn