Trans. Nonferrous Met. Soc. China 23(2013) 3206-3214
Potentiostatic corrosion protection technology under cavitation condition for 5083-H116 Al alloy
Seung-Jun LEE1, Young-Soo PARK2, Seong-Jong KIM1
1. Division of Marine Engineering, Mokpo Maritime University, Haeyangdaehak-ro 91Mokpo-Si, 530-729, Korea;
2. Division of Maritime Transportation Science, College of Maritime Sciences, Korea Maritime and Ocean University, 727 Taejong-ro, Yeongdo-gu, 606-791, Korea
Received 21 March 2013; accepted 14 June 2013
Abstract: This study aims to characterize the cavitation damage behavior of aluminum alloys in seawater and thus to enhance its cavitation resistance. Potentiostatic experiments were performed under various potential conditions to determine the optimum protection potentials, at which cavitation damage was suppressed. A potential range was selected for the experiment from -2.2 to -1.4 V, which corresponded to the concentration and activation polarization regions on the cathodic polarization curve of the 5083-H116 alloy. After the experiments, the current density-time behavior was investigated, and the degree of surface damage was observed using three-dimensional microscopy and scanning electron microscopy (SEM). The optimum protection potential was determined to be in the range of -1.9 to -1.5 V under which the cavitation damage was reduced significantly.
Key words: Al alloy; cavitation; potentiostatic experiment; seawater
1 Introduction
Compared to iron and steel, aluminum alloys have the advantages of lower density, higher specific strength, better machinability, and superior corrosion resistance. Therefore, lightweight aluminum alloys are being considered for use in aircraft, automobiles, railroad cars, and ships [1-3]. In particular, 5083-series aluminum alloys have excellent workabilities, e.g., machinability and weldability, and they are the most widely used in ship construction. Heat treatment and the incorporation of various additives have improved their corrosion resistance and thereby extended the life spans of ships made with them [4]. However, corrosion-erosion due to high speed and cavitation is still very likely to occur in harsh conditions such as marine environments. Cavitation damage that occurs in seawater results from composite actions, i.e., impact pressure produced by cavity collapse and corrosion by chloride ions present in the seawater. Many researchers studying this problem have individually analyzed and assessed cavitation damage and corrosion damage to infer a correlation between the two conditions [5,6]. However, a clear understanding of composite damage, such as cavitation- corrosion of materials exposed to corrosive environments and an assessment of damage behavior, is needed. Electrochemical corrosion behavior in cavitation environments should be assessed simultaneously; this is being done by several research groups [7-11]. The objective of the present investigation is to develop a potentiostatic corrosion-prevention technology using hydrogen gas generated by hydrogen overvoltage, where the hydrogen gas collides with the formed cavities at used amplitude, to minimize damage. The damage degree under the dynamic conditions of cavitation environments is compared with that under static conditions. The analysis result is used to determine the optimum potential conditions to prevent cavitation damage.
2 Experimental
The 5083-H116 aluminum alloy used in the present experiments was made by adding 4.74% Mg with Mn, Si, and Cr as very small amount elements to increase its strength and ductility. Its strength was further improved through cold working treatment. Table 1 shows the chemical composition of this alloy. A schematic diagram of the potentiostatic tests in cavitation environment is shown in Fig. 1. The test tank contained working electrodes (5083-H116), reference electrodes (Ag/AgCl), and counter electrode (platinum). Electrochemical cells were made using potentiostatic/galvanostatic equipment. The cavitation and electrochemical characteristics of the cells were assessed in seawater. In the cavitation experiments, vibration with constant amplitude of 10 μm was produced by piezoelectric effects in a vibration- generating system. The distance between the horn and the sample was maintained at 1 mm. Then, potentiostatic experiments were conducted under static conditions without cavitation to observe the current density behavior during the same time. After the experiments, the current density-time behavior and the surface damage degree were analyzed using three-dimensional microscopy and scanning electron microscopy (SEM).
Table 1 Chemical compositions of 5083-H116 Al alloy (mass fraction, %)
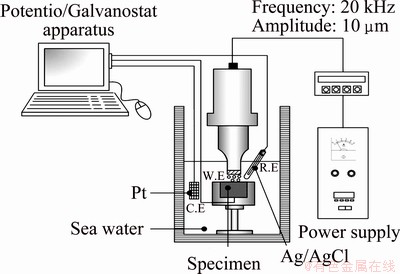
Fig. 1 Schematic diagram of potentiostatic experiment under cavitation condition
3 Results and discussion
3.1 Electrochemical tests
3.1.1 Cathodic polarization curves
To determine the appropriate potentiostatic test conditions, the cathodic polarization curves for 5083- H116 Al alloy [12] were plotted under different cavitation conditions by sweeping the potential from the open circuit potential down to -2.0 V with a scan rate of 2 mV/s. The potential from -1.4 V, which corresponds to concentration polarization from the dissolved oxygen reduction reactions, to -2.2 V, which corresponds to activation polarization, was selected as the potentiostatic condition (Fig. 2(a)). Under cavitation conditions (Fig. 2(b)), although the starting point of activation polarization was almost the same, i.e., at -1.6 V, the range of concentration polarization that corresponded to a corrosion-prevention section was much narrower. This was attributed to the dissolved oxygen reduction reaction that was suppressed by the cavitation impact pressure.

Fig. 2 Cathodic polarization curves of 5083-H116 Al alloy with and without cavitation
3.1.2 Current density-time curve in potentiostatic experiments
Figure 3 depicts the current density-time behavior in potentiostatic experiments with and without cavitation. Clear differences in current densities under different conditions were found from -1.6 to -1.4 V, which corresponded to concentration polarization due to the dissolved oxygen reduction reaction and activation polarization (Fig. 3(a1)). At -1.6 V, the current density rapidly increased from the beginning of immersion to around 1100 s, and then slowly decreased to a current density of 9.6256×10-4 A/cm2 at the end of the experiment. At -1.5 V, the current density also rapidly increased from the beginning of the experiment and remained generally stable from around 1700 s. Variations in current densities were large at -1.5 and -1.6 V because these potentials corresponded to turning points from concentration polarization due to the dissolved oxygen reduction reaction to activation polarization due to the hydrogen gas generation. At -1.4 V, where concentration polarization due to the dissolved oxygen reduction reaction occurred, current densities increased slightly at the beginning of immersion and slowly increased until the end of the experiment. From -1.9 to -1.7 V, current densities rapidly increased at the beginning and stabilized at a certain time. Current densities at the end of the experiments were 5.604×10-2, 2.987×10-2, and 1.169×10-2 A/cm2 for -1.9, -1.8, and -1.7 V, respectively. For the applied potentials from -2.2 to -2.0 V, current densities rapidly increased until around 140 s, and then leveled off to very large values until the end of experiment. As the applied potentials moved in the active direction, the current densities increased steadily. This was attributed to hydrogen overvoltage during activation polarization. The reactions of molecular hydrogen dominated over the reactions of atomic hydrogen. The current density at -1.6 V increased at the beginning, and began to slowly decrease at around 7000 s (Fig. 3(b1)); the magnitude was similar to that at -1.5 V. Whereas large changes in current densities were shown over time in static environments. Under this condition, the current densities tended to be constant and had almost the same values at the end of experiments. This was attributed to the removal of the oxidation protective films by the cavitation impact pressure. Over -1.9 to -1.7 V, the current density increased rapidly at the beginning of immersion, and then slowly decreased. From -2.2 to -2.0 V, the current densities increased rapidly at the beginning of immersion, and leveled off to nearly the same values. Generally, current densities increased as the applied potential moved in the active direction.
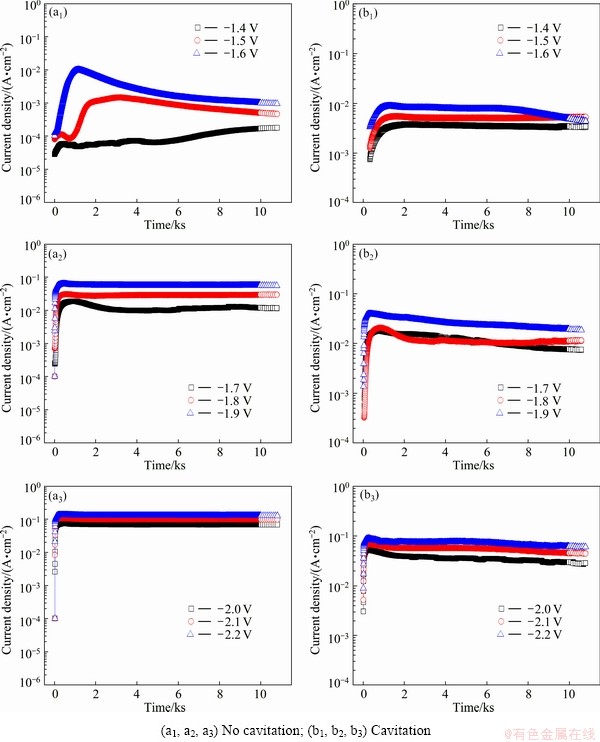
Fig. 3 Current density-time curves of 5083-H116 Al alloy during potentiostatic experiment with and without cavitation conditions
Figure 4 describes the current density values with and without cavitation. The current densities tended to increase as the potential decreased, whether or not cavitation was applied. The differences in current densities were smaller under the cavitation condition. In cases where cavitation was applied, differences between corrosion-prevention section and activation-reaction section were not clearly evident as the current densities continuously increased. Under both conditions, the lowest current density occurred when the applied potential was -1.4 V. When the applied potential values were -1.5 and -1. 6 V, activation polarization was at an early stage and damage due to cavitation was more influential than damage due to activation polarization. Consequently, higher static current densities were measured at -1.7 V or lower potentials and higher dynamic current densities were found at -1.6 V or higher potentials. This result was attributed to cavitation bubbles, which weakened the reactivity between the electrolyte solution and the material, and whose effects were larger at -1.7 V or lower potentials that were affected by hydrogen.
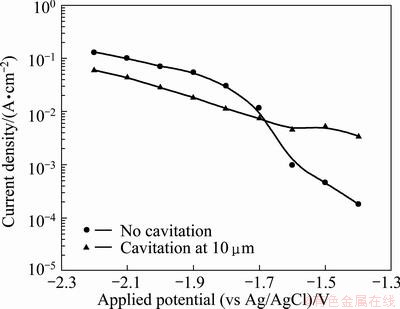
Fig. 4 Comparison of corrosion density for 5083-H116 Al alloy during potentiostatic experiment with and without cavitation
3.2 Surface characterization
3.2.1 Surface images after potentiostatic experiments
Figure 5 presents the surface images after the potentiostatic experiments when cavitation did and did not occur. Clean and undamaged surfaces were observed under static conditions for a concentration polarization potential of -1.4 V. From -2.2 to -1.6 V, which corresponded to activation polarization by hydrogen gas generation, as the potential decreased, the overvoltage increased and the amount of reduced hydrogen increased to promote activation reactions. Calcareous deposits were observed at potentials of -1.8 and -1.6 V. When the calcareous deposits were removed, crevice corrosion occurred at the interface between the calcareous deposits and the Al alloy. Calcareous deposits behave as corrosion-preventing coatings. They are formed by the precipitation of electrochemically generated CaCO3 or Mg(OH)2. If uneven calcareous coatings form, crevice corrosion will occur at the interface of Al alloy. Furthermore, galvanic cells will form between the Al alloy and the calcareous deposits, and thus deteriorate the corrosion resistance and mechanical properties. Under dynamic (cavitation) conditions, however, experiments at -1.4 V, which corresponded to a corrosion-prevention potential section under static conditions, caused greater damage than experiments at -1.6 or -1.8 V, where the specimens were affected by atomic hydrogen. Thus, although cavitation affected the samples almost identically at static conditions of -1.4 V which corresponded to concentration polarization, they were less damaged at -1.6 and -1.8 V due to the effects of the hydrogen gas generated by activation polarization: the hydrogen gas collided with cavities and reflected or dissipated them. In particular, the specimens showed good cavitation resistance, with less damage than cases where only cavitation was induced. Surface damage became deeper and more extensive as the potential decreased; the greatest damage was shown at -2.2 V by the effects of electrochemical corrosion and cavitation. This damage is likely to be due to the hydrogen evolution during activation polarization. Additionally, the material can become very sensitive to hydrogen-induced cracking that may cause brittle fractures under tensile stress. The damage increased because of the occurrence of the composite effects of the cavitation impact pressure imposed from the outside and excessive generated hydrogen gas [13-15]. Therefore, when the amount of hydrogen gas generated by activation polarization was greater than some threshold, the hydrogen gas did not greatly contribute to cavitation damage prevention.
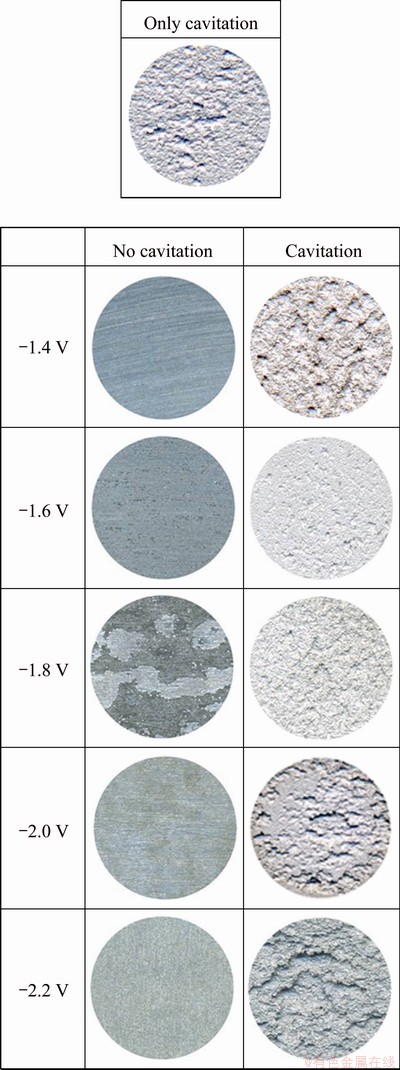
Fig. 5 Surface morphologies for 5083-H116 Al alloy after potentiostatic experiment with and without cavitation
3.2.2 Microstructure after potentiostatic experiments
Figure 6 shows the surfaces observed with SEM after the potentiostatic experiments with and without cavitation. Under static conditions, at -1.4 V which was a potential corresponding to concentration polarization, relatively clean surfaces were shown. Calcareous deposits formed at -1.6 V, and the Al alloy was damaged in areas from which calcareous deposits were removed. The damage was crevice corrosion derived from the formation of calcareous deposits. The corrosion in depth direction occurred because of pitting corrosion- generating mechanisms, such as self-propagating mechanism resulting from the accumulation of Cl- and H+ ions. Corrosion damage from hydrogen overvoltage was observed at -1.8 V or lower potentials. As polarization increased, the current densities increased and calcareous deposits formed on the surface of the cathode. At -1.8 V or lower potentials, the hydrogen generated by hydrogen overvoltage on the surface interfered with the formation of calcareous deposits [16]. When the potential decreased, the damaged surface was greater in the area and more broadly distributed. Under cavitation conditions, the samples were greatly damaged at -1.4 V due to the cavitation impact pressure. However, at -1.6 and -1.8 V, less damage was apparent compared with cases where only cavitation was applied, and cavitation resistance tended to increase. The hydrogen gas generated by the hydrogen overvoltage improved the cavitation resistance. This phenomenon did not persist at -2.0 V or lower potentials, showing rapid development of damage. This damage was attributed to excessive activation polarization, which occurred mainly with the effects of the electrochemical reactions. The effect of the hydrogen gas generated by the hydrogen overvoltage was the reason why only slight damage was shown at -1.6 and -1.8 V. Hydrogen gas is generated from the reduction of hydrogen ions during activation polarization, which is an electrochemical reaction. This hydrogen gas collides with cavities and reflects or dissipates them, thereby reducing the impact pressure delivered to the surface of Al alloy and protecting the Al alloy from damage.
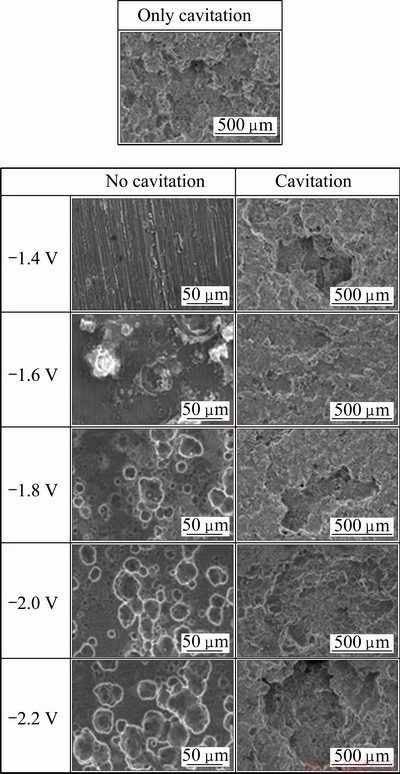
Fig. 6 SEM morphologies of 5083-H116 Al alloy after potentiostatic experiment with and without cavitation
3.2.3 Morphology after potentiostatic experiments
A three-dimensional analysis (Fig. 7) and a cross-sectional analysis (Fig. 8) showed the damaged surfaces after the potentiostatic experiments with and without cavitation. Under static conditions, -1.4 V corresponded to the potential range of concentration polarization by the dissolved oxygen reactions; only minute scratches produced from polishing were observed at this potential. However, at -1.6 V, partial surface damage caused by pitting corrosion was observed; this was because of the effects of atomic hydrogen generated by the activation reactions. Applied potentials not higher than -1.8 V corresponded to the potential range of activation polarization by hydrogen gas generation. At -2.2 and -2.0 V, as the potential moved in the active direction, activation polarization increased further and damage caused by the hydrogen gas generated through the reduction of hydrogen ions in the seawater solution was observed. As the potential moved in the active direction, pits tended to partially form and merge with each other to grow in depth. Unlike dissolution reactions, in activation polarization by hydrogen gas, the material does not participate firsthand in reactions, but depends on the rate of hydrogen reduction in the electrolyte solution. Hydrogen gas also affects the formation of calcareous deposits formed in the case of excessive cathodic polarization. The specimen was damaged at -1.4 V. At -1.6 V, less damage was observed compared with cases where only cavitation was induced; hydrogen gas generated by activation polarization collided with cavities and reflected or dissipated them. The surface damage became deeper and larger at -1.8 V and, between -2.2 and -2.0 V, the size of the pits increased as the potential decreased. This stemmed from the combined effects of electrochemical corrosion and cavities. The greatest damage was observed at the applied potential of -2.2 V, where the activation reactions were the most active.

Fig. 7 3D analysis of 5083-H116 Al alloy after potentiostatic experiment with and without cavitation
3.3 Maximum damage depth analysis
Figure 9 compares the current density and the maximum damage depth for the potentiostatic experiments with and without cavitation. Under static conditions (Fig. 9(a)), very slight damage around 3 μm occurred at the applied potentials of -1.4 and -1.5 V that corresponded to the turning points between concentration polarization and activation polarization. Corrosion damage of 9-12 μm occurred at -1.6 V when the specimen was affected by atomic hydrogen and at -1.7 to -1.8 V when the specimen began to be affected by molecular hydrogen. Thereafter, hydrogen overvoltage prevented the formation of calcareous deposits so that the damage depths rapidly increased at -1.9 V or lower potentials. A damage depth of 180.4 μm was measured at the applied potential of -1.4 V under cavitation condition (Fig. 9(b)). This potential corresponded to concentration polarization by dissolved oxygen reduction reaction. A reduction of damage was observed not only at the applied potential of -1.5 V, which corresponded to the turning point, but also during the activation polarization by atomic hydrogen (-1.8 to -1.6 V). Less damage occurred due to the hydrogen gas collided with the cavities generated by cavitation and reflected or dissipated them. Damage increased again from -1.9 V, when the material began to be affected by molecular hydrogen. The greatest damage, 266 μm, occurred at the lowest applied potential of -2.2 V, where electrochemical corrosion due to the generation of excessive hydrogen gas and damage due to cavities occurred simultaneously.

Fig. 8 Line profiles of 5083-H116 Al alloy after potentiostatic experiment with and without cavitation
Figure 10 compares the damage depth with and without corrosion-prevention cavitation conditions. A maximum damage depth of 151 μm was measured without corrosion-protection cavitation condition. A damage depth of 180 μm occurred at the applied potential of -1.4 V in cavitation condition, corresponding to concentration polarization by dissolved oxygen reduction reaction. Thereafter, as the potential decreased, much deeper damage occurred in cavitation condition compared with the cases where only cavitation experiments were conducted. Remarkably low damage values, i.e., 120 μm or lower, were found at -1.8 to -1.5 V. At these potentials, concentration polarization shifted into activation polarization and the generated hydrogen gas collided with cavities and reflected or dissipated them. Damage increased again from -1.9 V, at which the material began to be affected by molecular hydrogen, but the damage depths were smaller compared to that under non-protected cavitation condition. The material was damaged greatly at -2.0 V or lower potentials when electrochemical corrosion due to excessive overvoltage and damage due to cavities occurred simultaneously. In this study, potentiostatic experiments were conducted in diverse potential conditions to improve the cavitation resistance of an Al alloy in seawater. When these experiments were conducted under dynamic conditions in cavitation environments, damage by cavitation was small at potentials from -1.9 to -1.5 V. This was because of the effect of hydrogen gas generated in the range of activation polarization that collided with cavities formed by cavitation. Greater damage appeared at -2.0 V or lower potentials compared with cases where only cavitation experiments were conducted. This was because of damage by excessive activation polarization and by cavities. Consequently, the potential range of -1.9 to -1.5 V is the optimum protection potential range in which damage is minimized by cavitation.
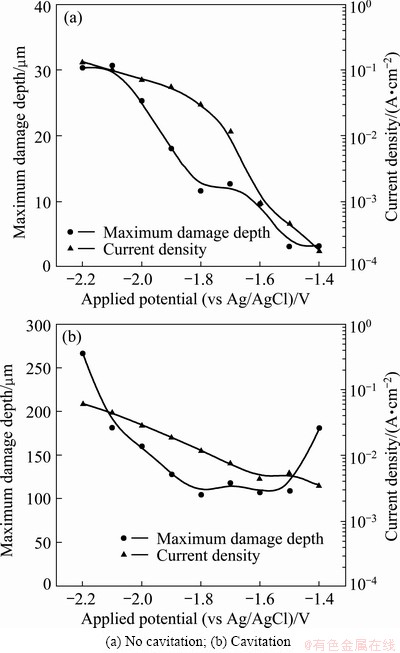
Fig. 9 Comparison of maximum damage depth and current density for 5083-H116 Al alloy after potentiostatic experiment with and without cavitation
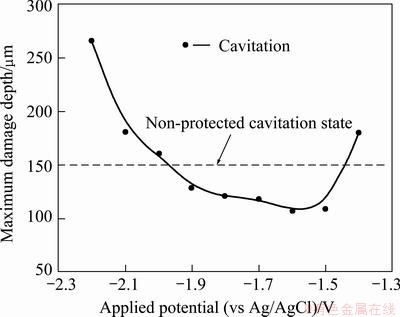
Fig. 10 Comparison of maximum damage depth for 5083-H116 Al alloy with and without cavitation
4 Conclusions
The potentiostatic experiments were conducted in diverse potential conditions to improve cavitation resistance of 5083-H116 Al alloy in seawater environments. When potentiostatic experiments were conducted in dynamic conditions of cavitation environments, damage by cavitation was shown to be small at potential of -1.9 to -1.5 V because of the effect of hydrogen gas generated in the potential range of activation polarization that collided with cavities formed by cavitation to reflect or dissipate the cavities. On the other hand, compared to only cavitation experiments, larger damage appeared under -2.0 V due to damage by excessive activation polarization and by cavities. Consequently, the optimum protection potentials for cavitation damage in cavitation environments were judged to be -1.9 to -1.5 V.
Acknowledgment
This study was sponsored by the Cooperative Promotion Center of Science & Technology of Jeonnam Technopark and Ministry of Education, Science and Technology (MEST) through “The research & development support of Jeonnam southwest science park”. We appreciate everyone who has supported and participated in this study.
References
[1] KIM S J, JANG S K, KIM J I. Investigation on optimum corrosion protection potential of Al alloy in marine environment [J]. Materials Science, 2008, 26(3): 779-786.
[2] SON I J, NAKANO H, OUE S, KOBAYASHI S, FUKUSHIMA H, HORITA Z. Effect of annealing on the pitting corrosion resistance of anodized aluminum-magnesium alloy processed by equal channel angular pressing [J]. Corrosion Science and Technology, 2007, 6(6): 275-281.
[3] SAKAIRI M, SHIMOYAMA Y, NAGASAWA D. Electrochemical random signal analysis during localized corrosion of anodized 1100 aluminum alloy in chloride environments [J]. Corrosion Science and Technology, 2008, 7(3): 168-172.
[4] CHUN Y J, CHUNG Y H, LEE Y H, SHIN M C. Effect of Zr addition on fracture toughness of 2048 high strength aluminum alloy [J]. The Korean Institute of Metals and Materials, 1990, 28(3): 217-244.
[5] HAN M S, LEE S J, JANG S K, KIM S J. Electrochemical and cavitation characteristics of Al thermal spray coating with F-Si sealing [J]. Corrosion Science and Technology, 2010, 9(6): 317-324.
[6] KIM S J, LEE S J. Investigation on electrochemical and cavitation characteristics of rudder materials for ship in sea water [J]. Corrosion Science and Technology, 2011, 10(3): 101-107.
[7] ZHENG Y, LUO S, KE W. Effect of passivity on electrochemical corrosion behavior of alloys during cavitation in aqueous solutions [J]. Wear, 2007, 262: 1308-1314.
[8] LATONA N, FETHERSTON P, CHEN A, SRIDHARAN K, DODD R. Wear-corrosion comparisons of passivating vs nonpassivating alloys inaerated 3.5% aqueous solutions of sodium chloride [J]. Corrosion, 2001, 57(10): 884-888.
[9] KWOK C T, CHENG F T, MAN H C. Laser surface modification of UNS S31603 stainless steel. Part I: Microstructures and corrosion characteristics [J]. Materials Science and Engineering A, 2000, 290: 55-73.
[10] NEVILLE A, HODGKIESS T. A study of the effect of liquid corrosivity in liquid-solid impingement of a cast iron and austenitic stainless steel [J]. British Corrosion, 1997, 32(3): 197-205.
[11] PARK J C, LEE S J, KIM S J. Cavitation and electrochemical characteristics using hydrogen overpotential method for ALBC3 alloy [J]. The Korean Institute of Surface Engineering, 2011, 44(6): 277-283.
[12] KIM S J, LEE S J, JANG S K, KIM K H. Evaluation of electrochemical characteristics of Al-Mg and Al-Mg-Si alloy in sea water [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: s881-s886.
[13] ELHOUD A M, RENTON N C, DEANS W F. Hydrogen embrittlement of super duplex stainless steel in acid solution [J]. International Journal of Hydrogen Energy, 2010, 35: 6455-6464.
[14] MICHLER T, NAUMANN J. Microstructural aspects upon hydrogen environment embrittlement of various bcc steels [J]. International Journal of Hydrogen Energy, 2010, 35: 821-832.
[15] MICHLER T, LEE Y, GANGLOFF R P, NAUMANN J. Influence of macro segregation on hydrogen environment embrittlement of SUS 316L stainless steel [J]. International Journal of Hydrogen Energy, 2009, 34: 3201-3209.
[16] RYU H J, LEE M H. Electro-deposit formed by mesh- electrodeposition technology on steel plate in seawater and their corrosion resistance [J]. The Corrosion Science Society of Korea, 2000, 29(4): 240-250.
5083-H116铝合金在气蚀条件下的恒电位腐蚀防护技术
Seung-Jun LEE1, Young-Soo PARK2, Seong-Jong KIM1
1. Division of Marine Engineering, Mokpo Maritime University, Haeyangdaehak-ro 91Mokpo-Si, 530-729, Korea;
2. Division of Maritime Transportation Science, College of Maritime Sciences, Korea Maritime and Ocean University, 727 Taejong-ro, Yeongdo-gu, 606-791, Korea
摘 要:研究铝合金5083-H116在海水中的气蚀损伤行为特征,以期提高合金的耐气蚀性能。通过在不同电位条件下进行的恒电位实验,确定抑制气蚀损伤的最优保护电位。实验在电位为-2.2~-1.4 V的范围内进行,这对应于铝合金5083-H116阴极极化曲线上的浓差极化和活化极化区域。对实验获得的电流密度—时间曲线进行研究,通过三维显微镜和扫描电子显微镜分析合金表面的损伤情况。结果表明,合金的最优保护电位为-1.9~-1.5 V,在此电位条件下,合金的气蚀损伤得到显著地减轻。
关键词:铝合金;气蚀;恒电位实验;海水
(Edited by Sai-qian YUAN)
Corresponding author: Seong-Jong KIM; Tel: +82-61-240-7226, Fax: +82-61-240-7201; E-mail: ksj@mmu.ac.kr
DOI: 10.1016/S1003-6326(13)62854-X