Mechanism of chlorinating lanthanum oxide and cerium oxide with ammonium chloride
来源期刊:中国有色金属学报(英文版)2003年第6期
论文作者:朱国才 李赋屏 肖明贵
文章页码:1454 - 1458
Key words:lanthanum; cerium; mechanism; chlorination; thermal decomposition
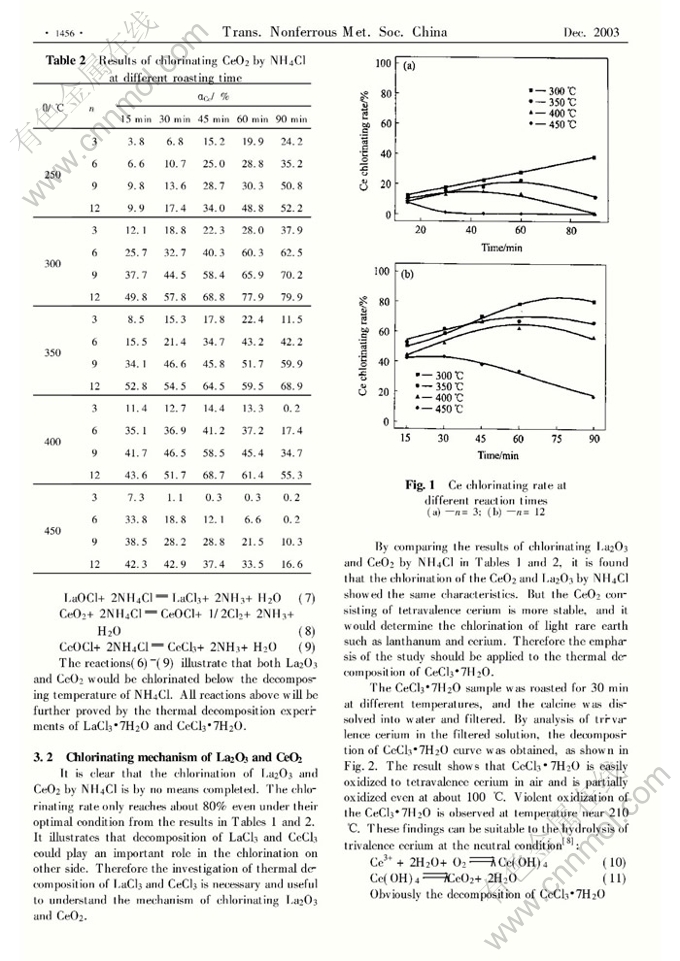
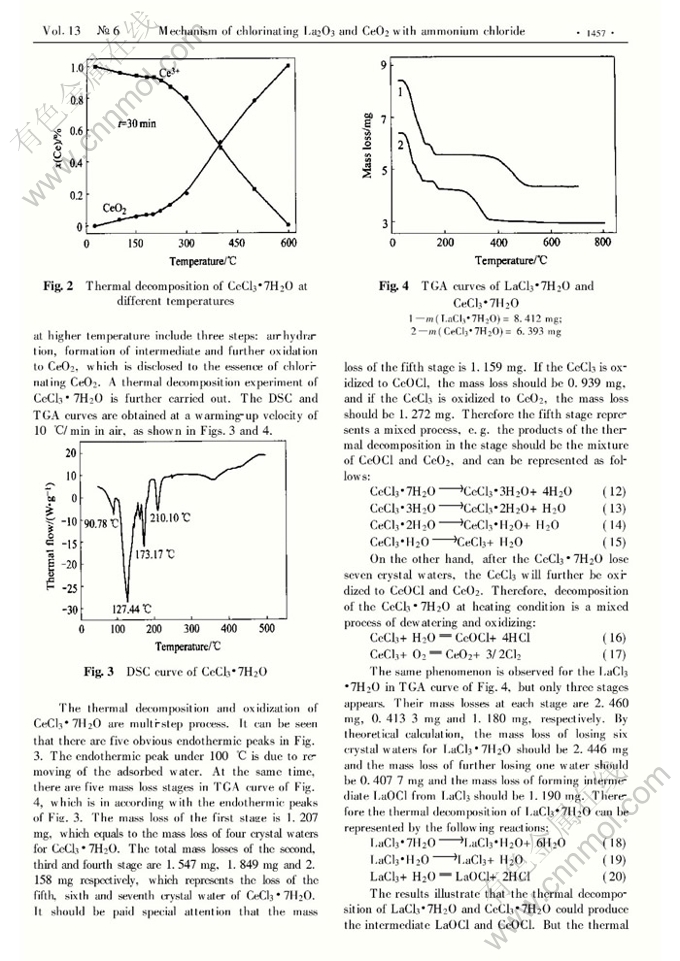
Abstract: Using ammonium chloride (NH4Cl) as a chlorinating agent, the effects of chlorinating temperature, chlorinating time and NH4Cl dosage on chlorination of La2O3 and CeO2, and the thermal decomposition of LaCl3·7H2O and CeCl3·7H2O were investigated. The results show that 80% of both La2O3 and CeO2 can be chlorinated at 300℃ for 90min, and have no advantage to chlorination of lanthanum and cerium oxides at higher temperature. The thermal decomposition of LaCl3 and CeCl3 is carried out to explore the mechanism of chlorinating lanthanum and cerium oxides. At the same time, the chlorination of lanthanum and cerium oxides is not devoted to the HCl decomposed from NH4Cl, but to NH4Cl directly taking part in the chlorination of La2O3 and CeO2. The lanthanum and cerium oxides in chlorination firstly form intermediate LaOCl and CeOCl, and then transfer to LaCl3 and CeCl3, finally to La2O3 and CeO2, respectively. The thermal decomposition analyses of LaCl3·7H2O and CeCl3·7H2O further prove the existence of the intermediates LaOCl and CeOCl. Therefore the chlorinating temperature and time should strictly be controlled when the lanthanum oxide and cerium oxide are chlorinated with NH4Cl. And over-dosage of NH4Cl should be also applied in the process of chlorination.