


Trans. Nonferrous Met. Soc. China 22(2012) 2014-2020
Biocorrosion property and cytocompatibility of calcium phosphate coated Mg alloy
XU Li-ping1, ZHANG Er-lin2, YANG Ke3
1. Guangzhou Research Institute of Nonferrous Metals, Guangzhou 510650, China;
2. Department of Materials Science and Engineering, Jiamusi University, Jiamusi 154007, China;
3. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 10 January 2012; accepted 27 June 2012
Abstract: Calcium phosphate coated Mg alloy was prepared. The phase constitute and surface morphology were identified and observed by X-ray diffractometer (XRD) and SEM. The results show that the coating is composed of flake-like CaHPO4·2H2O crystals. The corrosion resistance of the coated Mg alloy was measured by electrochemical polarization and immersion test in comparison with uncoated Mg alloy. Cytocompatibility was designed by observing the attachment, growth and proliferation of L929 cell on both coated and uncoated Mg alloy samples. The results display that the corrosion resistance of the coated Mg alloy is better than that of uncoated one. The immersion test also shows that the calcium phosphate coating can mitigate the corrosion of Mg alloy substrate, and tends to transform into hydroxyapatite (HA). Compared with uncoated Mg alloy, L929 cells exhibit good adherence, growth and proliferation characteristics on the coated Mg alloy, indicating that the cytocompatibility is significantly improved with the calcium phosphate coating.
Key words: biodegradable Mg; surface modification; corrosion; cytocompatibility
1 Introduction
Magnesium alloys have exhibited many excellent properties such as low density, high specific strength, good biocompatibility and mechanical properties close to that of natural bone. Moreover, magnesium alloys can be corroded or degraded in chloride-containing solutions [1] including human body fluid or blood plasma while present clinical metal materials like stainless steel and Ti alloys are un-degradable materials. Thus, magnesium alloys have attracted extensive attention in developing degradable medical metal materials. However, the rapid corrosion rate and the release of hydrogen gas during corrosion which results in a rapid increase in pH value and the subcutaneous gas bubbles limit their clinical application [2]. Therefore, it is necessary to improve initial corrosion resistance of Mg alloys by means of surface modification.
Concerning the implant materials, surface modification has to meet the requirements of both corrosion resistance and biocompatibility, meaning that the modification layer should be nontoxic and biocompatible. Calcium phosphates (Ca-P) contain the same chemical composition or structure as the mineral composition of natural bone and the release of Ca2+ and  during hydrolysis can be utilized in the course of forming new bone [3], so Ca-P coatings have been applied to the medical Ti alloys in order to improve their biocompatibility [4-7]. Recently, Ca-P coatings have been tried to prepare on pure Mg, AZ31, Mg-Zn and AZ91D by anodization [8], electrophoretic deposition [9-11] and biomimetic method [12,13]. And the results showed that the Ca-P coatings can improve the corrosion resistance of Mg substrates to different extends. While there has been limited work on depositing Ca-P on Mg alloy in phosphating baths. SINGH et al [14] reported that Ca-P coatings deposited through phosphating solution containing CaCl2, NaH2PO4 and Na2SiO3 on AZ31 and Mg-4Y. Upon drying and visual inspection, the substrate appeared to be coated inhomogeneously with a white precipitate. The results of FTIR and EDX confirmed the deposition of Ca, P and Si on both alloys and the coatings consisting of primarily biphasic mixtures of hydroxyapatite and β-TCP. The coating decreased the degradation of AZ31 whereas increased the degradation of Mg-4Y. MC3T3-E1 mouse osteoblasts test showed that silicate substituted CaP coatings were observed to increase the cell attachment on AZ31 but did not have a great effect on increasing cell attachment of Mg-4Y. A previous study has explored the successful preparation of a homogeneous Ca-P layer on a Mg-Mn-Zn alloy by phosphating treatment [15]. In this research, biocorrosion properties and cytocompatibility of a calcium phosphate coated Mg alloy in a phosphating bath mainly containing calcium dihydro phosphate were investigated.
during hydrolysis can be utilized in the course of forming new bone [3], so Ca-P coatings have been applied to the medical Ti alloys in order to improve their biocompatibility [4-7]. Recently, Ca-P coatings have been tried to prepare on pure Mg, AZ31, Mg-Zn and AZ91D by anodization [8], electrophoretic deposition [9-11] and biomimetic method [12,13]. And the results showed that the Ca-P coatings can improve the corrosion resistance of Mg substrates to different extends. While there has been limited work on depositing Ca-P on Mg alloy in phosphating baths. SINGH et al [14] reported that Ca-P coatings deposited through phosphating solution containing CaCl2, NaH2PO4 and Na2SiO3 on AZ31 and Mg-4Y. Upon drying and visual inspection, the substrate appeared to be coated inhomogeneously with a white precipitate. The results of FTIR and EDX confirmed the deposition of Ca, P and Si on both alloys and the coatings consisting of primarily biphasic mixtures of hydroxyapatite and β-TCP. The coating decreased the degradation of AZ31 whereas increased the degradation of Mg-4Y. MC3T3-E1 mouse osteoblasts test showed that silicate substituted CaP coatings were observed to increase the cell attachment on AZ31 but did not have a great effect on increasing cell attachment of Mg-4Y. A previous study has explored the successful preparation of a homogeneous Ca-P layer on a Mg-Mn-Zn alloy by phosphating treatment [15]. In this research, biocorrosion properties and cytocompatibility of a calcium phosphate coated Mg alloy in a phosphating bath mainly containing calcium dihydro phosphate were investigated.
2 Experimental
2.1 Sample preparation
Extruded Mg-Mn-Zn alloys were used with the composition (mass fraction) of 1.10% Mn, 1.05% Zn, 0.19% Al, ≤0.005% Ni, ≤0.005 Fe, ≤0.005% Cu and balance Mg. Samples with dimensions of 10 mm×10 mm ×15 mm were cut from the extruded alloy bar. After being ground and degreased, samples were embedded in epoxy resin with only one exposed side of 10 mm×10 mm. The sample surfaces were ground up to 1000 grit SiC paper, and then ultrasonically cleaned in alcohol for use.
The calcium phosphate coatings were produced using the following steps: ground up to 2000 grit SiC paper→rinsed with distilled water→degreased in an alkaline solution at 63 ℃ for 15 min→immersed in an acid solution→immersed in a treatment solution bath based on calcium dihydro phosphate for 15 min→dried in air.
2.2 Microstructure and phase composition
The microstructure of the calcium phosphate coating was observed on an SSX-550 SEM. The phase composition of the coating was identified by an X-ray diffractometer (XRD, D/MAX-RB, Riguka).
2.3 Electrochemical measurement
Electrochemical polarization tests were carried out at 37 ℃ in 350 mL of 0.9% NaCl using a CHI660A electrochemical workstation (CH Instruments Inc. USA). A three-electrode cell was used for the tests: the saturated calomel as a reference, a platinum electrode as the counter and the samples as the working electrode. All the tests were measured at a scanning rate of 0.3 mV/s.
2.4 Immersion test
Both the coated and uncoated samples were immersed in simulated body fluid (SBF) at 37 ℃ for 1, 4, 7 and 10 d, respectively. The ion concentration of SBF is as follows: 142.0 mmol/L Na+, 5.0 mmol/L K+, 1.5 mmol/L Mg2+, 2.5 mmol/L Ca2+, 147.8 mmol/L Cl-, 4.2 mmol/L 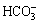 , 1.0 mmol/L
, 1.0 mmol/L 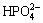 , 0.5 mmol/L
, 0.5 mmol/L  . The pH value of the SBF was measured before and after the immersion by a pH meter to calculate the change of the pH value. Before immersion, the pH of the SBF was adjusted to be 7.4.
. The pH value of the SBF was measured before and after the immersion by a pH meter to calculate the change of the pH value. Before immersion, the pH of the SBF was adjusted to be 7.4.
Before and after 10 d immersion, Fourier transform infrared spectrometry (FTIR, Spectrum one, Perkin- Elmer) was performed on the calcium phosphate coating.
After 10 d immersion, the surface morphology of the calcium phosphate coated Mg sample was also observed by SEM.
2.5 Cell experiment
Prior to the cell experiment, uncoated and coated samples were sterilized. After sterilization, the sample was placed into a well of a 48-well plate. L929 cells were seeded on the samples at a cell density of 10000 cell/mL. The plate was placed in a 5% (volume fraction) CO2 incubator for 1, 3 and 5d.
After a certain incubation period, the samples were fixed by 4% formaldehyde for 24 h, and then stained by means of immunofluorescence. The stained samples were observed using a TCS-SP2 laser scanning confocal microscope (LSCM). Cell counts were conducted at five different locations of each sample surface. A mean and a standard deviation were obtained from the measurements.
2.6 Statistical analysis
Statics analyses were performed using SPSS17.0. All the measurement results were expressed as the mean±SD. Statistical significant was defined as p<0.05.
3 Results and discussion
3.1 Microstructure and phase identification
The phase composition in the coating was analyzed by XRD. And the result is shown in Fig. 1. It can be seen that the coating mainly consists of CaHPO4·2H2O. Figure 2 displays the surface morphology of the coating. Lots of regular flake-like crystals can be observed on the coating. By combining XRD and SEM results, it can be concluded that the coating is mainly composed of flake-like CaHPO4·2H2O crystals.
3.2 Electrochemical polarization test
In order to assess the biocorrosion resistance of the calcium phosphate coated Mg alloy, electrochemical polarization tests in 0.9% NaCl were conducted on both uncoated and coated Mg alloy samples. The results are shown in Fig. 3. The electrochemical parameters obtained from Fig. 3 are summarized in Table 1. Compared with uncoated Mg alloy, corrosion potential (φcorr) of coated Mg alloy moves to more noble position, as shown in Fig. 3. The value of φcorr increases by 104 mV. Meanwhile, in comparison of uncoated Mg alloy, the corrosion current density (Jcorr) and corrosion resistance (Rp) of coated Mg alloy decrease and increases greatly, respectively, as shown in Table 1. Therefore, the noble φcorr, the low Jcorr and the high Rp obtained from the electrochemical test suggest that the calcium phosphate coating provides effective protection for the Mg alloy substrate and the calcium phosphate coated Mg shows better corrosion resistance than uncoated one.
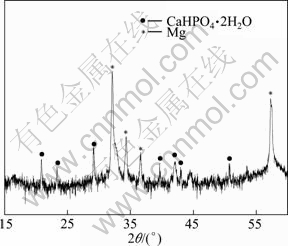
Fig. 1 XRD pattern of coating
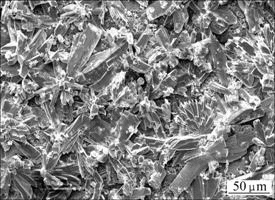
Fig. 2 SEM surface morphology of calcium phosphate coating

Fig. 3 Electrochemical polarization curves of Mg alloy samples with and without calcium phosphate coating
Table 1 Parameters of electrochemical polarization tests on Mg alloys in 0.9% NaCl solution

3.3 Immersion test in SBF
In order to further evaluate the biocorrosion resistance, the uncoated and coated Mg alloys were immersed in SBF for different periods. Figure 4 exhibits the pH value of the SBF with uncoated or coated Mg alloy against the immersion time. The pH value of the SBF with uncoated Mg alloy has the same tendency to SBF with coated Mg alloy: the pH value increases with the immersion time. However, the pH value of SBF with coated Mg alloy is lower than that of SBF with uncoated Mg alloy during the immersion. After 1 d immersion, the pH value of SBF with uncoated Mg alloy changes from initial value of 7.40 to 8.18 while the pH value of SBF with coated Mg alloy is about 8.01. In the following immersion, the difference in pH between the SBF with uncoated Mg alloy and coated Mg alloy is about 0.50.

Fig. 4 Change in pH value of SBF solutions containing Mg alloy with and without calcium phosphate coating
Mg is a relatively reactive metal. When Mg is contacted with aqueous solution like SBF, Mg will react with water to produce Mg(OH)2 and H2 gas. Chloride ions can transform the Mg(OH)2 into more soluble MgCl2 and release OH- [16-18]. The more severely the Mg corrodes, the more the OH- releases and the higher the pH value of the solution is. Thus, from the pH change shown in Fig. 4, it can be concluded that coated Mg releases less OH- than uncoated Mg during the immersion, and thus slows down the increase in pH value of the SBF. This also confirms that the coated Mg alloy shows better corrosion resistance than uncoated Mg alloy, and retards the corrosion of Mg alloy substrate.
Figure 5 illustrates the surface morphology of the calcium phosphate coated Mg alloy after 10 d immersion in SBF. No obvious difference in the surface morphology was observed by SEM compared with that before immersion (Fig. 2).
Figure 6 displays the FTIR spectra of the calcium phosphate coating before and after immersion in SBF for 10 d. According to Refs. [19-27], the FTIR spectrum of calcium phosphate coating before immersion is in accordance with the standard FTIR spectrum of CaHPO4·2H2O. The absorbance bands of 3543 cm-1 and 3478 cm-1 can be assigned to crystal water of CaHPO4·2H2O. The peak at 2382 cm-1 is related to 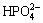 and the peak at 1650 cm-1 is the characteristics of H—O—H. The presence of bands at 1135 and 1217 cm-1 is related to the P=O stretching modes and the band at 1062 cm-1 is due to the P=O bending vibrations. The peaks at 987, 875 and 794 cm-1 can be attributed to P—O—P asymmetrical stretching modes. The peaks at 651, 577 and 527 cm-1 are related to (H—O—)P=O.
and the peak at 1650 cm-1 is the characteristics of H—O—H. The presence of bands at 1135 and 1217 cm-1 is related to the P=O stretching modes and the band at 1062 cm-1 is due to the P=O bending vibrations. The peaks at 987, 875 and 794 cm-1 can be attributed to P—O—P asymmetrical stretching modes. The peaks at 651, 577 and 527 cm-1 are related to (H—O—)P=O.
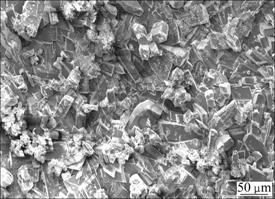
Fig. 5 SEM surface morphology of calcium phosphate coated Mg alloy immersed in SBF for 10 d
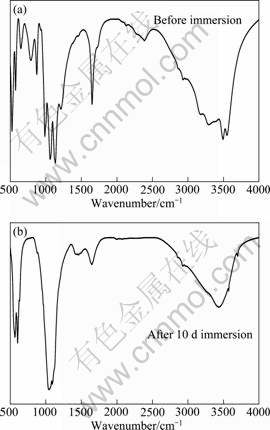
Fig. 6 FTIR analysis of calcium phosphate coating before and after immersion in SBF for 10 d
After 10 d immersion, many peaks related to the crystallized water of CaHPO4·2H2O and 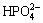 cannot be observed such as the peaks at 3543, 3478 and 2383 cm-1. Several peaks between 1500 and 500 cm-1 also disappear. Two obvious peaks appear at 566 and 602 cm-1. Two peaks at 1044 and 1090 cm-1 can also be seen which are corresponding to the vibrations of
cannot be observed such as the peaks at 3543, 3478 and 2383 cm-1. Several peaks between 1500 and 500 cm-1 also disappear. Two obvious peaks appear at 566 and 602 cm-1. Two peaks at 1044 and 1090 cm-1 can also be seen which are corresponding to the vibrations of  . In addition, two peaks at 630 and 3570 cm-1 can be found which can be attributed to the bending vibrations and stretching mode of OH- of HA. Based on the above analysis, it can be deduced that CaHPO4·2H2O tends to be transformed into HA phase after immersion in SBF for 10 d.
. In addition, two peaks at 630 and 3570 cm-1 can be found which can be attributed to the bending vibrations and stretching mode of OH- of HA. Based on the above analysis, it can be deduced that CaHPO4·2H2O tends to be transformed into HA phase after immersion in SBF for 10 d.
3.4 Cytocompatibility
Cytocompatibility is one of the important biological evaluations of biomaterials which can reflect the cell/biomaterial interactions. Figure 7 presents the morphology of the L929 cells cultured for 1, 3 and 5 d on both uncoated and coated Mg alloy by observation under LSCM. After 1 d culture, attached cells can be found on both uncoated and coated Mg alloys. A small number of round cells randomly distribute on uncoated Mg alloy (Fig. 7(a)), while more cells can be found on the coated Mg alloy and some cells are spindle-like or sail-like and contact with each other (Fig. 7(b)). After 3 d cultures, more cells can be observed on the uncoated Mg alloy. No obvious change in the cell morphology can be found (Fig. 7(c)). For coated Mg alloy, more cells can also be seen and the cells become big, meaning that the cells are spreading on the coated Mg alloy (Fig. 7(d)). After 5 d culture, no obvious change in the cell amount, morphology and distribution can be found on the uncoated Mg (Fig. 7(e)), while there are a large number of sail-like cells spreading on the coated Mg alloy (Fig. 7(f)).
Figure 8 shows the number of L929 cells attached to the Mg alloy samples against the culture time. For both uncoated and coated Mg alloys, the number of cells increases with the culture time. However, the number of cells on the coated Mg alloy is significantly (p<0.05) larger than that on the uncoated Mg alloy, meaning that the surface of coated Mg alloy is better for cell attachment, growth and proliferation.
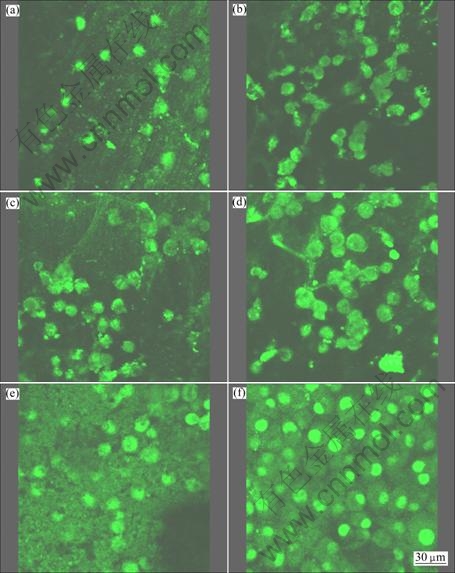
Fig. 7 Morphologies of L929 cells observed by LSCM after incubation for 1 d (a, b), 3 d (c, d), 5 d (e, f) on Mg alloy samples with and without calcium phosphate coating: (a), (c), (e) Without coating; (b), (d), (f) With calcium phosphate coating
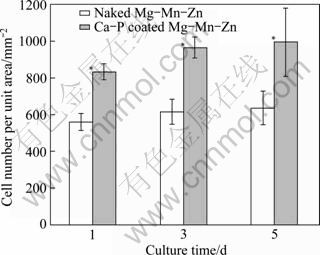
Fig. 8 Growth of L929 cells versus culturing time on Mg alloy samples with and without calcium phosphate coating, *p<0.05
The cellular responses to a material, such as attachment, proliferation and differentiation have a close relation to the chemical composition of the material [28]. For the coated Mg alloy, CaHPO4·2H2O can provide lots of Ca2+ which do good to absorbance of protein like fibronectin and vimentin which have a positive effect on cell adhesion and spreading [29]. Moreover, calcium phosphate coating can protect Mg alloy substrate from rapid corrosion to some extent and decrease the rapid increase of pH value of solutions, thus alkalescent conditions suitable to cell growth are kept. As a consequence, coated Mg alloy exhibits better cytocompatibility.
4 Conclusions
1) Calcium phosphate coated Mg alloy shows better corrosion resistance in both 0.9%NaCl solution and SBF in comparison with uncoated Mg alloy.
2) The CaHPO4·2H2O coating tends to be transform into HA phase after immersion of 10 d.
3) The cell culture test shows that coated Mg alloy has better cytocompatibility than uncoated Mg alloy.
Acknowledgements
Authors are thankful to Dr YU G N and Dr PAN F for the suggestions and help in the cell culture test.
References
[1] WITTE F, FISCHER J, NELLESEN J, CROSTACK H A, KAESE V, PISCH A, BECKMANN F, WINDHAGEN H. In vitro and in vivo corrosion measurements of magnesium alloys [J]. Biomaterials, 2006, 27(7): 1013-1018.
[2] WITTE F, KAESE V, HAFERKAMP H, SWITZER E, MEYER-LINDENBERG A, WIRTH C J, WINDHAGEN H. In vivo corrosion of four magnesium alloys and the associated bone response [J]. Biomaterials, 2005, 26(17): 3557-3563.
[3] HIGASHI S, YAMAMURO T, NAKAMURA T, IKADA Y, HYON S H, JAMSHIDI K. Polymer-hydroxyapatite composites for biodegradable bone fillers [J]. Biomaterials, 1986, 7(3): 183-187.
[4] PAITAL S R, DAHOTRE N B. Laser surface treatment for porous and textured Ca-P bio-ceramic coating on Ti-6Al-4V [J]. Biomedical Materials, 2007, 2: 274-281.
[5] REDEPENNING J, MCLSAAC J P. Electrocrystallization of brushite coatings on prosthetic alloys [J]. Chemistry of Materials, 1990, 2(6): 625-627.
[6] SHIRKHANZADEH M. Direct formation of nanophase hydroxyapatite on cathodically polarized electrodes [J]. Journal of Materials Science: Materials in Medicine, 1998, 9(2): 67-72.
[7] VIJAYARAGHAVAN T V, BENSALEM A. Electrodeposition of apatite coating on pure titanium and titanium alloys [J]. Journal of Materials Science Letters, 1994, 13(24): 1782-1785.
[8] HIRIMOTO S, SHISHIDO T, YAMAMOTO A, MARUYAMA N, SOMEKAWA H, MUKAI T. Precipitation control of calcium phosphate on pure magnesium by anodization [J]. Corrosion Science, 2008, 50: 2906-2913.
[9] SONY Y W, SHAN D Y, HAN E H. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application [J]. Materials Letters, 2008, 62(17-18): 3276-3279.
[10] SONG Y, ZHANG S, LI J, ZHAO C, ZHANG X. Electrodeoposition of Ca-P coatings on biodegradable Mg alloy: In vitro biomineralization behavior [J]. Acta Biomaterialia, 2010, 6(5): 1736-1742.
[11] WEN C L, GUAN S K, PENG L, REN C X, WANG X, HU Z H. Characterization and degradation behavior of AZ31 alloy surfac modified by bone like hydroxyapatite for implant applications [J]. Applied Surface Science, 2009, 255: 6433-6438.
[12] CUI F, YANG J, JIAO Y, YIN Q, ZHANG Y, LEE I S. Calcium phosphate coating on magnesium alloy for modification of degradation behavior [J]. Frontiers of Materials Science in China, 2008, 2(2): 143-148.
[13] WATERMAN J, PIETAK A, BIRBILIS N, WOODFIELD T, DIAS G, STAIGER M P. Corrosion resistance of biomimetic calcium phosphate coatings on magnesium due to varying pertreatment time [J]. Materials Science and Engineering B, 2011, 176: 1756-1760.
[14] SINGH S S, ROY A, LEE B, KUMTA P N. Aqueous deposition of calcium phosphates and silicate substituted calcium phosphates on magnesium alloys [J]. Materials Science and Engineering B, 2011, 176: 1695-1702.
[15] XU L, ZHANG E, YANG K. Phosphating treatment and corrosion properties of Mg-Mn-Zn alloy for bomedical application [J]. Journal of Materials Science: Materials in Medicine, 2009, 20(4): 855-867.
[16] ALTUN H, SEN S. Studies on the influence of chloride ion concentration and pH on the corrosion and electrochemical behaviour of AZ63 magnesium alloy [J]. Materials and Design, 2004, 25(7): 637-643.
[17] BALDWIN K R, BRAY D J, HOWARD G D, GARDINER R W. Corrosion behaviour of some vapour deposited magnesium alloys [J]. Materials Science and Technology, 1996, 12: 937-943.
[18] MAKAR G L, KRUGER J. Corrosion of magnesium [J]. International Materials Reviews, 1993, 38(3): 138-153.
[19] ASHOK M, KALKURA S, SUNDARAM N, ARIVUOLI D. Growth and characterization of hydroxyapatite crystals by hydrothermal method [J]. Journal of Materials Science: Materials in Medicine, 2007, 18(5): 895-898.
[20] JINAWATH S, SUJARIDWORAKUN P. Fabrication of porous calcium phosphates[J]. Materials Science and Engineering C, 2002, 22(1): 41-46.
[21] JOSHI V S, JOSHI M J. FTIR spectroscopic, thermal and growth morphological studies of calcium hydrogen phosphate dihydrate crystals [J]. Crystal Research and Technology, 2003, 38(9): 817-821.
[22] MUN B B, JUNG K H, CHAI Y G, KIM H K. The influence of bioactive inorganic materials on osteopontin expression in rat calvarial osteoblast culture [J]. Bulletin of The Korean Chemical Society, 2007, 28(4): 652-656.
[23] ZHANG H, WANG Y, YAN Y, LI S. Precipitation of biocompatible hydroxyapatite whiskers from moderately acid solution [J]. Ceramics International, 2003, 29(4): 413-418.
[24] LIU Rong-fang, XIAO Xiu-feng, LIN Lan-yun, CHEN Gu-yong. Studies on hydrothermal electrodeposition of hydroxyapatite coatings [J]. Chemial Journal of Chinese Universities, 2004, 25(2): 304-308. (in Chinese)
[25] MO Li-rong, LI Yu-bao, LV Guo-yu, LI Ji-dong, YANG Wei-hu. Study on nano-hydroxyapatite crystals synthesized under different conditions [J]. Chemical Research and Application, 2006, 18(3): 230-234. (in Chinese)
[26] XIAO Xiu-feng, TANG Xiao-lian, GAO Yan-jiao, XU Yi-zhan, LIU Rong-fang. Effect of technological parameters on composition and structure of electrodeposited calcium phosphate coatings [J]. Materials Protection, 2005, 38(12): 29-32. (in Chinese)
[27] YUAN Jun, WU Yuan, ZHENG Qi-xin. Synthesis of hydroxyapatite nano-rods/whiskers by precipitation in impinging streams [J]. Orthopaedic Biomechanics Materials and Clinical Study, 2006, 3(5): 1-3. (in Chinese)
[28] SUGIMOTO T, KANATANI M, KANO J. Effects of high calcium concentration on the functions and interactions of osteoblastic cells and monocytes and on the formation of osteoclast-like cells [J]. Journal of Bone Mineral Research, 1993, 8: 1445-1452.
[29] NI S Y, LIN K L, CHANG J, CHOU L. Beta-CaSiO3/beta-Ca3(PO4)2 composite materials for hard tissue repair: In vitro studies [J]. Journal of Biomedical Materials Research Part A, 2008, 85(1): 72-82.
磷酸钙表面改性镁合金的生物腐蚀性能及细胞相容性
徐丽萍1,张二林2,杨 珂3
1. 广州有色金属研究院,广州510650;
2. 佳木斯大学 材料科学与工程系,佳木斯 154007;
3. 中国科学院 金属研究所,沈阳 110016
摘 要:在镁合金表面制备磷酸钙涂层,利用X射线衍射仪确定涂层的相组成。用扫描电镜观察涂层的微观形貌。结果表明,涂层由板条状的CaHPO4·2H2O晶体组成。采用电化学测试和浸泡实验研究磷酸钙改性镁合金的生物腐蚀性能,并与未改性合金进行对比。通过观察L929细胞在材料表面的粘附生长状况来评价材料的生物相容性。电化学测试结果表明,磷酸钙改性镁合金比未改性合金显示出更好的耐腐蚀性能。浸泡实验表明,磷酸钙涂层可以减缓合金的腐蚀,且在浸泡过程中磷酸钙涂层发生了向羟基磷灰石(HA)的转变。与未改性合金相比,L929细胞在磷酸钙改性镁合金表面显示出良好的粘附、生长和分化特征,表明磷酸钙改性能明显提高基体合金的细胞相容性。
关键词:生物可降解镁;表面改性;腐蚀;细胞相容性
(Edited by LI Xiang-qun)
Corresponding author: XU Li-ping; Tel: +86-20-61086357; E-mail: lipingxu2008@gmail.com
DOI: 10.1016/S1003-6326(11)61422-2