文章编号:1004-0609(2012)06-1785-06
基于最小Gibbs自由能原理的CaO-SiO2炉渣和Si-Ca金属两相体系平衡热力学模型
王 飞1,郭汉杰1,杨学民2
(1. 北京科技大学 冶金与生态工程学院, 北京 100083;
2. 中国科学院 过程工程研究所 多相复杂系统国家重点实验室,北京 100190)
摘 要:基于封闭平衡体系Gibbs自由能最小原理对精炼Si液中杂质Ca的炉渣-金属体系建立热力学模型,由该热力学模型得到Si液中Ca的摩尔分数xCa与CaO-SiO2二元渣系CaO的摩尔分数xCaO的关系和文献报道的实验结果吻合良好,即0.3<xCaO<0.4较有利于Si液脱Ca。热力学模型计算结果表明:Si液中Si和Ca的活度分别与SiO2和CaO质量作用浓度的比值符合炉渣-金属组元活度之间的关系式,且计算结果比文献报道的数据精度更高。因此,基于炉渣离子-分子共存理论的质量作用浓度可与经典的炉渣活度一样表征CaO-SiO2二元渣系组元的反应能力。由建立的热力学模型计算结果确定的反应[Ca]+0.5(SiO2)=(CaO)+0.5[Si]的标准摩尔反应Gibbs自由能变化表达式为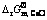 =-89 532.7-21.8T (J/mol)。
=-89 532.7-21.8T (J/mol)。
关键词: CaO-SiO2渣系;Si-Ca熔体;最小自由能原理;热力学模型;离子-分子共存理论;Si液精炼;活度
中图分类号:TF61 文献标志码:A
Thermodynamic model of CaO-SiO2
binary slags equilibrated with Si-Ca binary metal system based on minimized Gibbs free energy principle
WANG Fei1, GUO Han-jie1, YANG Xue-min2
(1. School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing,
Beijing 100083, China;
2. State Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100190, China)
Abstract: A thermodynamic model was developed for a close system of CaO-SiO2 binary slags equilibrated with Si-Ca binary metal system based on the minimized Gibbs free energy principle of a close system, in which the reaction ability of components in slags was presented by the mass action concentration according to the ion and molecule coexistence theory. The calculated relationship between mole fraction of Ca, xCa, in silicon melt and mole fraction of CaO, xCaO, in CaO-SiO2 slags is in good agreement with the reported results, i.e., the optimal composition range for xCaO is 0.3<xCaO< 0.4. The calculated relationship between the ratio of Si activity in melt to mass action concentration of SiO2 in slags and the ratio of Ca activity in melt to mass action concentration of CaO in slags follows the intrinsic relation, while the calculated results has higher accuracy than that from the reported results based on experiments. Therefore, the mass action concentrations of components in CaO-SiO2 slags can be successfully used to present reaction ability like component activity in slags. The determined standard molar Gibbs free energy change for reaction [Ca]+0.5(SiO2)= (CaO)+0.5[Si] from the developed thermodynamic model is 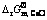 =-89 532.7-21.8T (J/mol) with reliable feasibility.
=-89 532.7-21.8T (J/mol) with reliable feasibility.
Key words: CaO-SiO2 binary slags; Si-Ca metal system; minimized free energy principle; thermodynamic model; ion- molecule coexistence theory; silicon refining; activity
大量使用化石燃料产生的污染物和温室气体排放以及化石燃料储量有限性使得世界各国投入大量人力和财力研究清洁能源。太阳能作为古老的清洁能源之一无疑受到广泛重视。高纯硅或太阳能级硅(w(Si)>99.999 9%)是高效利用太阳能的重要基本材料。太阳能级硅以w(Si)>99.7%的金属硅为原料。含有少量Ca、Al等杂质元素的金属硅主要采用CaO-SiO2-Al2O3渣系精炼获得;其中脱除Ca的基本原理为Si液中的杂质元素Ca被CaO-SiO2二元渣系中SiO2氧化为CaO进入渣中。为此,KUME等[1]和WEISS等[2]研究CaO-SiO2二元渣系和Si-Ca二元金属体系在1 823~ 1 873 K温度范围平衡条件下Ca在该渣系与Si液间的分配规律;MIKI等[3-4]研究了Si-Ca二元金属体系中Ca的活度aCa和Ca活度系数γCa。ZHANG等[5-7]、张鉴[8]以及YANG等[9]采用炉渣离子-分子共存理论研究CaO-SiO2二元炉渣结构单元或离子对(相当于传统冶金物理化学中的组元)的质量作用浓度(相当于传统物理化学中的活度)。结果表明[5-9],CaO-SiO2二元渣系中结构单元的质量作用浓度Ni可与组元的活度ai建立良好对应关系。
由于前人对CaO-SiO2二元渣系和Si-Ca二元金属体系的平衡反应研究报道尚不多,且CaO-SiO2二元渣系和Si-Ca二元金属体系平衡反应的标准摩尔Gibbs反应自由能变化 也彼此不一致,缺少公认的表达式[10, 13-15]。因此,本文作者在分别采用炉渣离 子-分子共存理论表征CaO-SiO2二元渣系组元反应能力和基于Henry定律表征Si-Ca二元金属体系中Ca的活度aCa的基础上,基于该炉渣-金属构成的封闭平衡体系自由能最小原理,研究CaO-SiO2二元渣系和Si-Ca二元金属系两相间反应热力学平衡。其目的不仅是将已证明[5–9]适用于钢铁冶金成分范围内的炉渣离子-分子共存理论拓展到Si精炼成分范围内CaO-SiO2二元渣系的组元反应能力表征,同时为CaO-SiO2二元渣系和Si-Ca二元金属体系组成的平衡体系提供更完整、更精确的反应热力学数据和标准摩尔Gibbs反应自由能变化通用表达式。
也彼此不一致,缺少公认的表达式[10, 13-15]。因此,本文作者在分别采用炉渣离 子-分子共存理论表征CaO-SiO2二元渣系组元反应能力和基于Henry定律表征Si-Ca二元金属体系中Ca的活度aCa的基础上,基于该炉渣-金属构成的封闭平衡体系自由能最小原理,研究CaO-SiO2二元渣系和Si-Ca二元金属系两相间反应热力学平衡。其目的不仅是将已证明[5–9]适用于钢铁冶金成分范围内的炉渣离子-分子共存理论拓展到Si精炼成分范围内CaO-SiO2二元渣系的组元反应能力表征,同时为CaO-SiO2二元渣系和Si-Ca二元金属体系组成的平衡体系提供更完整、更精确的反应热力学数据和标准摩尔Gibbs反应自由能变化通用表达式。
1 模型的建立
1.1 体系及平衡描述
CaO-SiO2二元渣系和Si-Ca二元金属体系的炉 渣-金属界面处反应[1, 10]可表征为
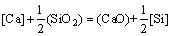 (1)
(1)
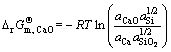 (J/mol) (2)
(J/mol) (2)
式中:a表示各反应物或产物的活度。
由于CaO-SiO2二元渣系和Si-Ca二元金属体系构成的Si液中脱除Ca的精炼过程是封闭的,根据相律分析可知,当温度T一定且CaO-SiO2二元渣系和Si-Ca二元金属体系两相系中任意两个组元的浓度(或活度)确定后,则该封闭体系的平衡状态亦确定,即CaO-SiO2二元渣系和Si-Ca二元金属体系达到热力学平衡[12]的充分必要条件为该封闭体系的总Gibbs自由能最小。
1.2 基于炉渣离子-分子共存理论的CaO-SiO2二元渣系中结构单元反应能力表征
1.2.1 CaO-SiO2二元渣系的炉渣离子-分子共存理论假定
根据文献[5-9]报道的炉渣离子-分子共存理论的假设,采用炉渣离子-分子共存理论表征CaO-SiO2二元渣系的结构单元或离子对的质量作用浓度时的主要假设可概括如下:
1) 与Si液平衡的CaO-SiO2二元渣系中结构单元由Ca2+和O2-简单离子、SiO2简单分子及CaO·SiO2等为代表的硅酸盐复杂大分子组成。每个结构单元在熔渣中占有其独立位置,但由同一组元离解的阳离子和阴离子参与形成复杂大分子反应时以如(Me2++O2-)所表征的离子对形式参与反应;
2) 由离子对和简单分子生成复杂大分子的反应保持化学动态平衡;
3) 在一定温度和浓度范围内,与Si-Ca二元金属体系反应的CaO-SiO2二元渣系中所有结构单元具有连续性;
4) 形成复杂大分子的化学反应服从质量作用定律。
1.2.2 CaO-SiO2二元渣系中结构单元或离子对及其质量作用浓度
根据前已述及的CaO-SiO2二元渣系的炉渣离子-分子共存理论[5-9]的基本假设以及在Si液精炼的温度范围内(1 823~1 873 K)的CaO-SiO2二元渣系的相图,CaO-SiO2二元渣系中存在两种复杂大分子2CaO·SiO2和CaO·SiO2。根据炉渣离子-分子共存理论[5-9],炉渣中结构单元的质量作用浓度Ni可由下式表征:
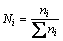 (-) (3)
(-) (3)
对炉渣中以(Me2++O2-)表征的离子对,其质量作用浓度NMeO可表征为
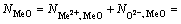
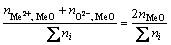 (-) (4)
(-) (4)
式中: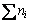 为炉渣-金属反应平衡时一定质量的炉渣中所有结构单元的摩尔数总和,mol;ni为炉渣-金属反应平衡时一定质量的炉渣中结构单元i的摩尔数,mol。
为炉渣-金属反应平衡时一定质量的炉渣中所有结构单元的摩尔数总和,mol;ni为炉渣-金属反应平衡时一定质量的炉渣中结构单元i的摩尔数,mol。
本研究以100 g炉渣质量为基进行计算。显然,基于炉渣离子-分子共存理论[5-9]计算的CaO-SiO2二元渣系中结构单元的Ni的物理化学意义为摩尔分数,且炉渣中所有结构单元的N i之和为1.0,即
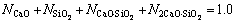 (-) (5)
(-) (5)
以纯物质为标准态,摩尔分数为浓度单位的经典冶金物理化学所定义的炉渣组元的活度ai与炉渣组元的质量作用浓度Ni的物理意义一致,所以在表征上述封闭体系中各炉渣组元的纯物质标准摩尔Gibbs自由能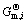 可采用质量作用浓度Ni替代活度ai,则炉渣的总Gibbs自由能(Gi)可表达为
可采用质量作用浓度Ni替代活度ai,则炉渣的总Gibbs自由能(Gi)可表达为
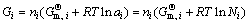 (J) (6)
(J) (6)
1.3 Si-Ca二元金属体系中组元的活度
Si-Ca二元金属体系中Si为溶剂,其活度服从Raoult定律,Si的活度因子γSi以1.0计;Si液中的Ca的活度aCa服从Henry定律。研究表明[3]:Si-Ca二元金属体系Ca活度因子γCa可表征为
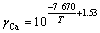 (-) (7)
(-) (7)
因此,Si-Ca二元金属体系中,aSi≈1.0,aCa=xCaγCa。
1.4 CaO-SiO2二元渣系和Si-Ca二元金属体系构成的封闭体系标准摩尔Gibbs自由能表征
对任意的多相封闭体系,当温度和压力确定后,体系中所有反应达到热力学平衡的充分必要条件是该封闭体系总的标准摩尔Gibbs自由能最小,且该体系中的各元素物质量守恒[12]。由一定质量CaO-SiO2二元渣系和一定质量Si-Ca二元金属体系组成的封闭体系的总Gibbs自由能Gs可表征为
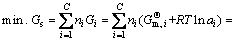
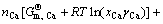
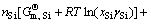
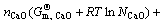


 (J) (8)
(J) (8)
式中:min. Gs为Gs最小值;C为体系的总的组元个数,6,(-);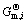 为组元i或结构单元i的标准摩尔Gibbs自由能,J/mol;ni 为100 g炉渣或100 g Si液中组元i达到渣-金反应平衡时的摩尔数,mol;ai 为炉渣中组元i或Si液中组元i以纯物质为标准态的活度,(-);xi 为Si液中组
为组元i或结构单元i的标准摩尔Gibbs自由能,J/mol;ni 为100 g炉渣或100 g Si液中组元i达到渣-金反应平衡时的摩尔数,mol;ai 为炉渣中组元i或Si液中组元i以纯物质为标准态的活度,(-);xi 为Si液中组
元i的摩尔分数,即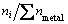 ,(-);Ni 为炉渣中各结构单元的质量作用浓度,即摩尔分数
,(-);Ni 为炉渣中各结构单元的质量作用浓度,即摩尔分数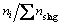 ,(-);
,(-);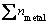 为100 g Si液中组元的总摩尔数,mol;
为100 g Si液中组元的总摩尔数,mol;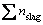 为100 g炉渣中结构单元的总摩尔数,mol,即
为100 g炉渣中结构单元的总摩尔数,mol,即
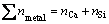 (mol) (9)
(mol) (9)
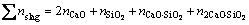 (mol) (10)
(mol) (10)
式(10)中的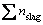 与式(4)中的
与式(4)中的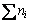 的数值和物理意义完全一样。由100 g 的CaO-SiO2二元渣系和100 g 的Si液构成的封闭体系中独立元素为Ca、Si和O且物质量守恒。Ca,Si和O的物质量守恒表达式为
的数值和物理意义完全一样。由100 g 的CaO-SiO2二元渣系和100 g 的Si液构成的封闭体系中独立元素为Ca、Si和O且物质量守恒。Ca,Si和O的物质量守恒表达式为
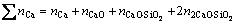 (mol) (11)
(mol) (11)
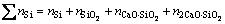 (mol) (12)
(mol) (12)
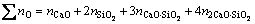 (mol)(13)
(mol)(13)
显然,式(8)为目标函数和式(9)~(13)为约束条件。当式(8)中Gs达到最小值时,该封闭体系反应达到平衡。本研究采用Lingo 6.0软件求解了1 823 K和1 873 K时,不同Ca含量Si-Ca二元金属体系对应的平衡CaO-SiO2二元渣系中所有结构单元的质量作用浓度Ni。采用的CaO-SiO2二元渣系和Si-Ca二元金属体系构成的封闭体系中所有物相纯物质标准摩尔Gibbs自由能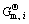 如表1[11]所列。
如表1[11]所列。
2 结果与讨论
2.1 模型计算结果
采用前文所述的热力学模型,对文献[1-2]报道的1 823 K和1 873 K温度时CaO-SiO2二元渣系对应Si-Ca二元金属体系平衡组分进行计算,结果如表2所列。为了比较,将文献[1-2]报道的Si-Ca二元金属体系平衡实验数据也总结于表2。
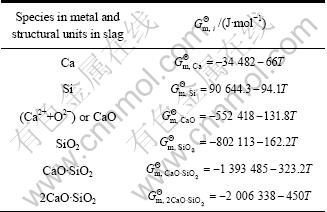
表1 CaO-SiO2二元渣系和Si-Ca二元金属体系中各组元或结构单元的标准摩尔Gibbs自由能数据[11]
Table 1 Standard molar Gibbs free energies of all possible species or structural units in both CaO-SiO2 binary slags and Si-Ca binary metal system[11]
文献[1-2]报道和本研究计算的温度为1 823 K和1 873 K时Si液中Ca的摩尔分数xCaO随CaO-SiO2二元渣系中CaO的摩尔分数xCaO(xCaO<0.5)的变化关系如图1所示。由图1可知,本研究计算的结果和文献[1-2]报道的实测结果吻合较好。随温度升高,CaO- SiO2二元渣系中相同CaO摩尔分数xCaO对应的平衡Si液中Ca摩尔分数xCa减小。这表明,温度由1 823 K提高到1 873 K,有助于该二元渣系对Si中杂质元素Ca的脱除。当该渣系中xCaO<0.4时,增加炉渣中xCaO对Si液中xCa影响较小;炉渣中xCaO>0.4时,增加炉渣中xCaO,Si液中xCa明显增加。这表明,在高CaO含量的炉渣成分范围内,增加CaO含量不但不能脱除Si液中Ca,反而会明显增加杂质Ca在Si液中的含量。虽然CaO含量较低的该二元渣系有利于脱除Si液中杂质Ca,但过低的CaO使得该渣系二元简单碱度(w(CaO)/w(SiO2))减少,炉渣黏度增大,进而使得该渣系炉渣流动性变差,降低炉渣-金属间反应速率,还会造成炉渣和Si液难以分离。显然,0.3<xCaO<0.4较有利于该二元渣系脱除Si液中杂质元素Ca。
表2 CaO-SiO2 二元渣系和Si-Ca二元金属体系平衡实验实测数据和模型计算结果的比较[1-2]
Table 2 Comparison of measured compositions of CaO-SiO2 binary slags equilibrated with Si-Ca binary metal system and calculated results[1-2]
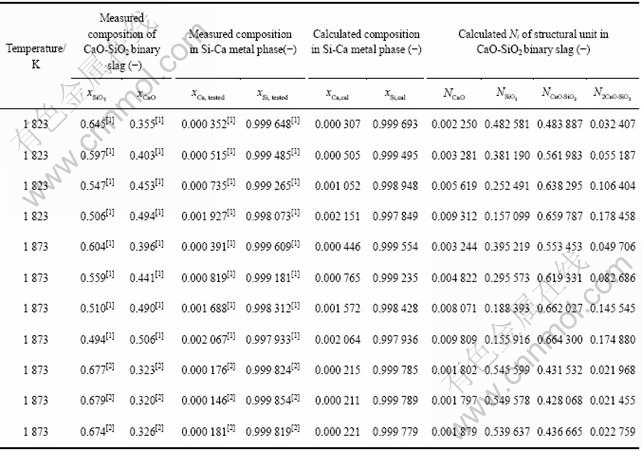
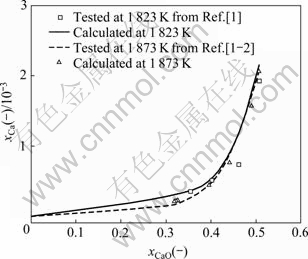
图1 温度为1 823 K和1 873 K时CaO-SiO2二元渣系和Si-Ca二元金属体系平衡体系中CaO的摩尔分数xCaO与Si液中Ca的摩尔分数xCa之间关系
Fig. 1 Relationship between mole fraction of CaO in CaO-SiO2 binary slags and mole fraction of Ca in Si-Ca binary metal system at 1 823 K and 1 873 K
2.2 CaO-SiO2二元渣系和Si-Ca二元金属体系中组元反应能力或活度间的关系及炉渣-金属反应的
2.2.1 CaO-SiO2二元渣系和Si-Ca二元金属体系中组元反应能力或活度间的关系
前已述及,基于炉渣离子–分子共存理论[5–9]所定义的炉渣结构单元的质量作用浓度Ni与经典冶金物理化学所定义的炉渣组元活度ai一样表征炉渣组元反应能力,因此,式(1)所示的CaO-SiO2二元渣系脱除Si-Ca二元金属体系中Ca的反应平衡常数K可表征为
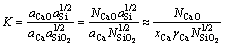 (–) (14)
(–) (14)
式(14)可变型为
 (–) (15)
(–) (15)
显然,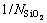 与aCa/NCaO应存在良好的二次方关系。采用本研究得到的
与aCa/NCaO应存在良好的二次方关系。采用本研究得到的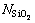 和NCaO分别与
和NCaO分别与 和
和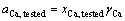 得到的
得到的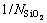 与aCa/NCaO分别示于图2。显然,计算的
与aCa/NCaO分别示于图2。显然,计算的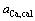 与采用实测数据表征
与采用实测数据表征 十分吻合。这表明基于炉渣离子–分子共存理论[5–9]计算得到的Si液精炼成分适用范围内的CaO-SiO2二元渣系中CaO和SiO2的质量作用浓度Ni可准确表征该渣系的反应能力。根据计算的
十分吻合。这表明基于炉渣离子–分子共存理论[5–9]计算得到的Si液精炼成分适用范围内的CaO-SiO2二元渣系中CaO和SiO2的质量作用浓度Ni可准确表征该渣系的反应能力。根据计算的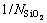 与aCa/NCaO拟合出的K1 823和K1 873分别为5 061和4 322.7。
与aCa/NCaO拟合出的K1 823和K1 873分别为5 061和4 322.7。
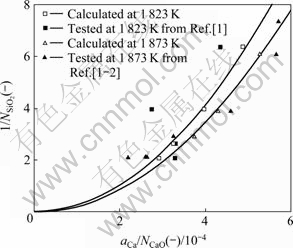
图2 温度为1 823 K和1 873 K时CaO-SiO2二元渣系和Si-Ca二元金属体系平衡体系中aCa/NCaO与aSi/NSiO2的关系
Fig. 2 Relationship between aCa/NCaO and aSi/NSiO2 with aCa, cal and aCa, tested presenting aCa in close system composed of CaO-SiO2 binary slags and Si-Ca binary metal system at 1 823 and 1 873 K
2.2.2 炉渣-金属间反应的标准摩尔反应Gibbs自由能变化
根据2.2.1节确定的K1 823和K1 873,采用CaO-SiO2二元渣系脱除Si液中Ca反应的标准摩尔Gibbs反应能变化 与平衡常数K的关系
与平衡常数K的关系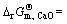
 ,可确定出1823 K和1873 K时的
,可确定出1823 K和1873 K时的 。据此回归出的
。据此回归出的 表达式为
表达式为
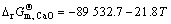 (J/mol) (16)
(J/mol) (16)
本研究得到的温度在1 823~1 873 K范围 与文献[13–15]报道相近温度范围内的
与文献[13–15]报道相近温度范围内的 分别示于图3。由图3可知,在1 823~1 873 K温度范围内,WAKASUGI和SANO[13]确定的
分别示于图3。由图3可知,在1 823~1 873 K温度范围内,WAKASUGI和SANO[13]确定的 最大,本研究得到
最大,本研究得到 的次之,MIN和SANO[15]确定的
的次之,MIN和SANO[15]确定的 较小,SATA等[14]确定的
较小,SATA等[14]确定的 最小。但MIN和SANO[15]与WAKASUGI和SANO[13]确定的
最小。但MIN和SANO[15]与WAKASUGI和SANO[13]确定的 与本研究得到的
与本研究得到的 差别不大。本研究得到的
差别不大。本研究得到的 与WAKASUGI和SANO[13]确定的
与WAKASUGI和SANO[13]确定的 最大差值为9 303.32 J/mol,SATA等[14]与WAKASUGI和SANO[13]确定的
最大差值为9 303.32 J/mol,SATA等[14]与WAKASUGI和SANO[13]确定的 最大差值30 111 J/mol,MIN和SANO[15]与WAKASUGI和SANO[13]确定的
最大差值30 111 J/mol,MIN和SANO[15]与WAKASUGI和SANO[13]确定的 最大差值14 392.7 J/mol。WAKASUGI和SANO[13]通过实验指出,较大的
最大差值14 392.7 J/mol。WAKASUGI和SANO[13]通过实验指出,较大的 值较为合理。本研究确定的
值较为合理。本研究确定的 与WAKASUGI和SANO[13]通过实验确定的
与WAKASUGI和SANO[13]通过实验确定的 较为接近,且本研究建立的模型对各组元活度描述更为准确,因此其结果更加可靠。
较为接近,且本研究建立的模型对各组元活度描述更为准确,因此其结果更加可靠。
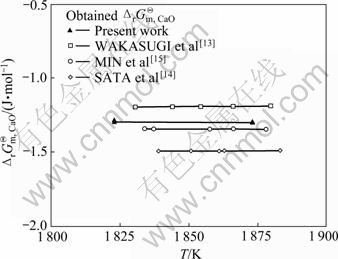
图3 [Ca]+0.5(SiO2)=(CaO)+0.5[Si]反应的标准摩尔反应Gibbs自由能变化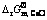 的比较
的比较
Fig. 3 Comparison of obtained standard molar reaction Gibbs free energy change 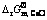 for [Ca]+0.5(SiO2)=(CaO)+ 0.5[Si] by present study and other researchers
for [Ca]+0.5(SiO2)=(CaO)+ 0.5[Si] by present study and other researchers
3 结论
1) 基于平衡体系Gibbs自由能最小原理对精炼Si液中杂质Ca的炉渣-金属体系建立了热力学模型,由热力学模型得到的Si液中Ca的摩尔分数xCa与CaO-SiO2二元渣系CaO的摩尔分数xCaO的结果和文献报道的实验结果良好吻合,即0.3<xCaO<0.4较有利于Si液脱Ca。
2) 建立的热力学模型计算结果表明,Si液中Si和Ca的活度分别与SiO2和CaO质量作用浓度的比值符合炉渣-金属间组元活度之间的关系式,且计算结果比文献报道的数据精度更高。因此,基于炉渣离子–分子共存理论的质量作用浓度可与经典的炉渣作用活度一样表征CaO-SiO2二元渣系组元的反应能力。
3) 由热力学模型计算结果确定的炉渣-金属间反应[Ca]+0.5(SiO2)=(CaO)+0.5[Si]的标准摩尔反应自由能变化表达式为 =-89 532.7-21.8T J/mol。
=-89 532.7-21.8T J/mol。
REFERENCES
[1] KUME K, MORITA K, MIKI T, SANO N. Activity measurement of CaO-SiO2-AlO1.5-MgO slags equilibrated with silicon alloys[J]. ISIJ International, 2000, 40(6): 561-566.
[2] WEISS T, SCHWERDTFEGER K. Chemical equilibria between silicon and slag melts[J]. Metallurgical and Materials Transactions B, 1994, 25(4): 497-504.
[3] MIKI T, MORITA K, SANO N. Thermodynamic properties of aluminum, magnesium, and calcium in molten silicon[J]. Metallurgical and Materials Transactions B, 1999, 29(5): 1043-1049.
[4] MIKI T, MORITA K, SANO N. Thermodynamic properties of Si-Al, -Ca, -Mg binary and Si-Ca-Al, -Ti, -Fe ternary alloys[J]. Materials Transactions (JIM), 1999, 40(10): 1108-1116.
[5] ZHANG Jian. The application of the law of mass action in combination with the coexistence theory of slag structure to the multicomponent slag systems[J]. Acta Metallurgica Sinica, 2001, 14 (3): 177-190.
[6] ZHANG Jian. Applicability of mass action law to sulphur distribution between slag melts and liquid iron[J]. Journal of University of Science and Technology Beijing, 2002, 9 (2): 90-98.
[7] ZHANG Jian. Application of annexation principle to the study of thermodynamic properties of ternary molten salts CaCl2-MgCl2-NaCl[J]. Rare Metals, 2004, 23 (3): 209-213.
[8] 张 鉴. 冶金熔体的计算热力学[M]. 北京: 冶金工业出版社, 2007.
ZHANG Jian. Computational thermodynamics of metallurgical melts and solutions[M]. Beijing: Metallurgical Industry Press, 2007.
[9] YANG Xue-min, JIAO Jin-sha, DING Ru-cai, SHI Cheng-bin, GUO Han-jie. A thermodynamic model for calculating sulphur distribution ratio between CaO-SiO2-MgO-Al2O3 iron making slags and carbon saturated hot metal based on the ion and molecule coexistence theory[J]. ISIJ International, 2009, 49 (12): 1828-1837.
[10] WANG Xin-guo, DING Wei-zhong, TANG Kai, JIANG Guo-chang, XU Kuang-di. Experimental thermodynamic research on equilibrium between silicon alloy and SiO2-CaO- Al2O3 melt[J]. Transactions of Nonferrous Metals Society of China, 2001, 11(4): 535-539.
[11] BARIN I. Thermochemical data of pure substances[M]. 3rd ed (partⅠ,Ⅱ). Weinheim: Federal Republic of Germany, 1995.
[12] WHITE W B, JOUNSON S M, DANTZIC G B. Chemical equilibrium in complex mixtures[J]. The Journal of Chemical Physics, 1958, 28(5): 751-755.
[13] WAKASUGI T, SANO N. Re-evaluation of standard free energy of formation of CaO[J]. Metallurgical and Materials Transactions B, 1989, 20(3): 431-433.
[14] SATA T, SASAMOTO T, MATSUMOTO K. High-temperature vaporization of calcium oxide[J]. High Temperatures-High Pressures, 1982, 14(4): 399-408.
[15] MIN D J, SANO N. Determination of standard free energies of formation of Ca3P2 and Ca2Sn at high temperatures[J]. Metallurgical and Materials Transactions B, 1988, 19(3): 433-439.
(编辑 李艳红)
收稿日期:2011-01-05;修订日期:2011-05-19
通信作者:杨学民,博士;电话:010-82622893;E-mail: yangxm71@hom.ipe.ac.cn