
Hafnium carbide structural foams synthesized from polymer precursors
FAN Hai-bo, YANG Hong, N. K. RAVALA, H. C. WIKLE III, R. H. ZEE, B. A. CHIN
275 Wilmore Labs Auburn University, Auburn, AL 36849-5341, USA
Received 20 April 2006; accepted 30 June 2006
Abstract: Hafnium carbide (HfC) was applied in space and aerospace due to its ultra high melting temperature, high specific strength and moderate oxidation resistance. A novel synthesizing method was used to produce low density and high strength HfC structural foams through the thermolysis and pyrolysis of Hf containing polymer precursors (mixing of hafnium trifluoroacetylacetonate and epoxy) under vacuum atmosphere. The X-ray diffraction analysis shows that the produced foam is primarily composed of HfC containing 9%-10% HfO2. Several polymer powder compaction methods were used to improve the mechanical properties of HfC foam. Compression strengths of 200 MPa are achieved for HfC foams with density of 1.9 g/cm3 (total porosity about 85%). The proposed methodology of synthesizing HfC foam has the advantages of simple, inexpensive and less production time than alternate methods.
Key words: Hafnium carbide; mechanical properties; polymer precursors; foam
1 Introduction
HfC has the highest melting point of any known binary alloy, approximately at 3 900 ℃. HfC has good thermo-mechanical and thermo-chemical properties at high temperatures. These properties make HfC foam as an appealing candidate for spacecraft and ultra high temperature applications at temperatures of above 1 600 ?C. The reaction forming method was used by PALMISIANO et al[1] to produce high density HfC foams, which involved placing a glassy carbon preform on a bed of molten Hf metal or Hf metal-silicon alloy. A lot of studies were conducted to use the chemical vapor deposition (CVD) to develop HfC protective coatings on different substrates. EMIG et al[2], WUNDER et al[3-5] and POPOVSKA et al[6] investigated the CVD of hafnium carbide coatings for carbon fiber reinforced carbon (CFC) and carbon fiber reinforced silicon carbide (C/SiC). SOURDIAUCOURT et al[7, 8] and ODDISOURDIAUCOURT et al[9] studied the depos- ition of hafnium carbide on the carbon foam substrate. Recently, SAYIR [10] used the CVD technique to produce monolithic hafnium carbide and tantalum carbide. The vacuum plasma spray (VPS) technique was used by AGARWAL et al[11] to study the near net forming of hafnium-based ceramic components.
The reaction forming method involves creating HfC foam by infiltrating Hf at temperatures of above 2 100 K. VPS requires very high electrical currents and the created HfC deposit is a highly dense material which is undesirable for space applications. CVD was widely studied to produce HfC. CVD, however, is usually a very slow and expensive process requiring hours to produce micrometers of material. In the present investigation, a simple approach requiring less production time was studied to produce low density HfC structural foams from polymer precursors[12].
2 Experimental
Hf containing polymers (hafnium trifluoroacetyl-
acetonate and epoxy) were mixed to create polymer preform samples. Polymer preforms were heat treated under vacuum using an initial low temperature thermolysis, and then, the heat-treatment was stopped and the samples were removed from the furnace. The samples were pulverized, sieved, and the powder produced was cold pressed into polymer cylinders. These polymer cylinders were then heat-treated with a second produce of HfC foam. The thermolysis step at lower temperatures produced a preliminary foam structure, and
the pyrolysis step at higher temperatures removed gaseous products and completed the HfC conversion process.
3 Results and discussion
3.1 Particle size distribution of polymer powder
Polymer preforms were removed from the vacuum furnace after the initial thermolysis and pulverized with a mortar and pestle. Fig.1 shows the particle size distribution diagram of produced polymer powder. From Fig.1, the bar graph uses the secondary Y axis, and each bar height indicates the population of particular particle size group as a percentage of the total particles. The curve known as a “cumulative percent smaller curve” in Fig.1 uses the major Y axis. For instance, the point (84.35 μm, 80%) on the curve should be read as 80% of the particles are smaller than 84.35 μm. The particle size distribution shown in Fig.1 has a near symmetric distribution. The mean diameter of measured particle volume is 56.44 ?m. Fig.2 shows photo of polymer pellet be cold pressed from the polymer powder of Fig.1. The average bulk density measured of 5 samples of polymer cylinders is (1.13±0.02)g/cm3.

Fig.1 Particle size distribution of powder made by mortar and pestle

Fig.2 Photo of polymer pellet (cylinder) made by polymer powder under cold pressing
3.2 Characterization of HfC foam sample
Fig.3 shows photo of an HfC foam sample after heat-treatment completely. The HfC sample maintains a cylindrical shape and has grown in the vertical direction.

Fig.3 Photo of HfC foam created from polymer cylinders
Fig.4 shows the broken surface microstructure of a compression tested HfC foam sample as shown in Fig.3. From Fig.4, this surface shows evenly distributed, equiaxed half cells as well as connected particles in the foam structure. The major structural element of these HfC foams is the connected HfC particles. The equiaxed half cells hypothesized are formed from large polymer particles that accumulate gases and expand during heating.

Fig.4 Surface microstructure of HfC foam sample shown in Fig.3
X-ray diffraction (XRD) was used to confirm the conversion of HfC of samples. Compared the XRD pattern with the File (PDF) database of JCPDS powder diffraction, the material is primarily composed of HfC. No residual graphite peak is found. Small amounts of HfO2 are evident in the XRD pattern. Based upon the relationship between XRD peak height ratio and mass fraction of HfO2, the content of estimated HfO2 is 9%-10%.
3.3 Effects of processing on compression strength
The polymer sample pulverized using an auto-
pulverizer (Labtechnics dish and puck crusher) ranges was also investigated. Three pulverizing times were investigated (5, 15 and 30 min) to obtain different particle sizes and distributions. Figs.1 , 5, 6 and 7 show the particle size distributions of powder produced by the different processing methods.
Compared Figs.1, 5, 6 and 7, the particle size distribution of powder made by mortar & pestle is more symmetric(Fig.1), and the powder made by auto-pulve-
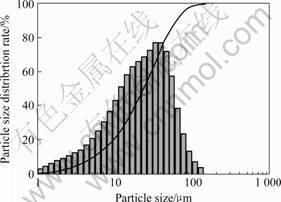
Fig.5 Particle size distribution of powder pulverized for 5min using Labtechnics pulverizer

Fig.6 Particle size distribution of powder pulverized for 15 min using Labtechnics pulverizer
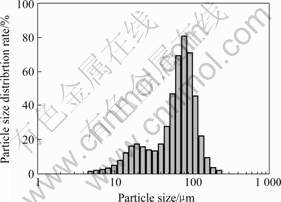
Fig.7 Particle size distribution of powder pulverized for 30 min using Labtechnics pulverizer
rizing for 5 min (Fig.5) is more symmetric than that for 15 min (Fig.6) and 30 min(Fig.7). The powder made by auto-pulverizing for 15 min(Fig.6) and 30 min(Fig.7) show bimodal distributions and a larger particle size than that for 5 min(Fig.5). The larger particle size is hypothesized due to particle agglomeration from the longer pulverizing period and the temperature rise of the pulverizer bowl (from room temperature to 50-80 ℃). Table 1 lists the particle size measurements of powders made by different methods and processing times.
Table 1 Particles size analysis results of polymer powder made by different methods and processing times

Fig.8 shows the effects of different powder processing conditions on HfC foam density and compression strength. For each group in Fig.8, the density and compression strength results were measured from 5 to 10 samples. Fig.8 reveals that the powder made by auto-pulverizing for 5 min(Fig.5) and yielded foams with higher density and higher compression strength than that by mortar & pestle(Fig.1). Compared Fig.5 with Fig.1, both powders have a near symmetric size distribution, but the average particle size of the powder made by auto-pulverizing for 5 min is smaller than that by mortar & pestle. Since a smaller powder particle size favors a higher packing density, this can explain the higher final HfC foam density of powder made by auto-pulverizing for 5 min. The increasing foam density also leads to higher foam compression strength.
Fig.8 also shows the effect of pulverizing times on

Fig.8 Effect of powder processing methods on foam density and compression strength
the foam density and compression strength. When the pulverizing time increases from 5 min to 15 min or 30 min, the foam density increases from 1.35 g/cm3 to 1.8-1.9 g/cm3, and the foam compression strength is improved from 25 MPa to 200 MPa, which can be explained by the particle size distribution difference between powder pulverized for 5 min and 15 min or 30 min. Powders pulverized for 15 min or 30 min have a non-uniform bimodal distribution, which enables the finer particles to fill the holes between the larger particles during cold pressing and creates the higher packing density and foam density. Again, an increasing foam density lead to higher foam compression strength.
4 Conclusions
The HfC foam was produced through the thermolysis and pyrolysis of Hf containing polymers under vacuum atmosphere. The XRD pattern of the HfC foam samples show that the manufactured foam material is primarily composed of HfC. Small amounts of HfO2 are foud evidently in the XRD pattern. The HfC foams produced have evenly distributed foam cells with diameter size up to 100 mm.
The HfC foam mechanical properties are improved by optimizing the polymer powder compaction method. The HfC foams with a density of 1.8-1.9 g/cm3 (total porosity about 85%), and a foam compression strength of about 200 MPa are achieved. Particle size and size distribution introduced by different powder processing methods and parameters change, which leads to HfC foam density and compression strength change. A bimodal size distribution of small and large particle sizes increase the foam density and the compression strength.
References
[1] PALMISPALMISIANO M N, JAKUBENAS K J, BARANWAL R. Reaction-forming Method for Producing Near Net-shape Refractory Metal Carbides[P]. U.S. Patent # 6764620, 2004.
[2] EMIG G, SCHOCH G, WORMER O. Chemical vapor deposition of hafnium carbide and hafnium nitride[J]. Journal de Physique IV: Proceedings 3, 1993: 535-540.
[3] WUNDER V, POPOVSKA N, EMIG G. Chemical vapor deposition of hafnium carbide on carbon substrate[J]. Proceedings- Electrochemical Society, 1997, 97-25: 608-615.
[4] WUNDER V K, POPOVSKA N, EMIG G. Study of hafnium carbide growth by CVD from in situ chlorinated hafnium[J]. Proceed- ings-Electrochemical Society, 1999, 98-23: 264-267.
[5] WUNDER V K, POPOVSKA N, EMIG G. Hafnium carbide as a barrier in multilayer coatings by chemical vapor deposition (CVD)[J]. Journal de Physique IV: Proceedings 9, 1999: 509-516.
[6] POPOVSKA N, HELD D, WUNDER V, GERHARD H, EMIG G. Chemical vapour deposition of pyrolytical carbon and graded C/SiC/Si-films at atmospheric pressure[J]. Proceedings-Electroche- mical Society, 1999, 98-23: 407-412.
[7] SOURDIAUCOURT P, DERRE A, DELHAES P, DAVID P. Thermodynamical and experimental conditions of hafnium carbide chemical vapour deposition[J]. Journal de Physique IV: Proceedings 9, 1999: 373-380.
[8] SOURDIAUCOURT P, DERRE A, DELHAES P, DAVID P. Hafnium carbide deposit on a carbon foam[J]. Chocs, 2001, 24: 51-61.
[9] ODDISOURDIAUCOURT P, DERRE A, DELHAES P, DAVID P. Mechanical reinforcement of carbon foam by hafnium carbide deposit[J]. Journal de Physique IV: Proceedings 9, 1999: 1187-1194.
[10] SAYIR A. Carbon fiber reinforced hafnium carbide composite[J]. Journal of Materials Science, 2004, 39: 5995-6003.
[11] AGARWAL A, MCKECHNIE T, STARETT S, OPEKA M M. Near net shape forming of hafnium-based ceramic components: synthesis and characterization[A]. Elevated Temperature Coatings: Science and Technology IV[C]. New Orleans, United States: LA, 2001: 302-315.
[12] FAN Hai-bo. HfC structural foams synthesized from polymer precursors[D]. Auburn: Auburn University, 2005.
(Edited by LI Yan-hong)
Corresponding author: FAN Hai-bo; Tel: +01-334-844-3322; Fax: +01-334-844-3400; E-mail: bchin@eng.auburn.edu