J. Cent. South Univ. (2012) 19: 1622-1628
DOI: 10.1007/s11771-012-1185-0
Influence factors on thermal conductivity of ammonia-water nanofluids
YANG Liu(杨柳), DU Kai(杜垲), ZHANG Xiao-song(张小松)
School of Energy and Environment, Southeast University, Nanjing 210096, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: In order to investigate the mechanism of nanoparticles enhancing the heat and mass transfer of the ammonia-water absorption process, several types of binary nanofluids were prepared by mixing Al2O3 nanoparticles with polyacrylic acid (PAA), TiO2 with polyethylene glycol (PEG 1000), and TiN, SiC, hydroxyapatite (noodle-like) with PEG 10000 to ammonia-water solution, respectively. The thermal conductivities were measured by using a KD2 Pro thermal properties analyzer. The influences of surfactant and ammonia on the dispersion stabilities of the binary nanofluids were investigated by the light absorbency ratio index methods. The results show that the type, content and size of nanoparticles, the temperature as well as the dispersion stability are the key parameters that affect the thermal conductivity of nanofluids. For the given nanoparticle material and the base fluid, the thermal conductivity ratio of the nanofluid to the ammonia-water liquid increases as the nanoparticle content and the temperature are increased, and the diameter of nanoparticle is decreased. Furthermore, the thermal conductivity ratio increases significantly by improving the stabilities of nanofluids, which is achieved by adding surfactants or performing the proper ammonia content in the fluid.
Key words: binary nanofluids; ammonia-water; thermal conductivity; size effect; dispersion stability
1 Introduction
Ammonia-water absorption refrigerator has superiority in utilizing the waste-heat and low-grade heat source and they have drawn renewed attention with the growing awareness of the dual threats of global warming and ozone depletion. However, the drawbacks of ammonia-water absorption refrigeration, such as the heavy equipment, the low coefficient of performance (COP) and the hazard in safety, restricted its broad application. Therefore, many researches have been performed to improve its performance. Generally, there are three methods to enhance the efficiency of heat and mass transfer: the mechanical treatment, the chemical treatment and nanotechnology [1].
Over the past several decades, nanoparticles have triggered an explosion of scientific and industrial interest due to their outstanding characteristics. It has long been recognized that the suspensions of solid particles in liquids provide useful advantages in heat transfer fluid [2-3]. The working fluids have the limitation of heat transfer performance, thus solid particles are dispersed in the working fluids to improve their thermal properties or heat transfer characteristics [4]. Consequently, many experiments have been performed on the application of nanofluid in ammonia-water absorption. LEE et al [5] found that the absorption rate and heat transfer rate of ammonia-water nanofluid with 0.002% Al2O3 nano- particles were 29% and 18% higher than those of the ammonia-water without nanoparticles, respectively, and the ammonia-water nanofluid with 0.002% Al2O3 nanoparticles was the optimal candidate for ammonia- water absorption performance enhancement. Yang et al [6] carried out the comparative experiments on the falling ?lm absorption between ammonia-water and ammonia-water with various kinds of nanoparticles. It was found that the effective absorption ratio can be increased by 70% and 50% with Fe2O3 and ZnFe2O4 nano?uid, respectively, when the initial ammonia mass fraction is 15%.
The enhancement of nanoparticles on the heat transfer of single-species fluid has been studied by many researchers. Choi [7] reported that the thermal conductivity of the base fluid increased up to 40% by adding a little amount of nanoparticles and nanotubes. To explain Choi’s experimental results, Keblinski et al [8] suggested the potential mechanisms for thermal conductivity enhancement such as Brownian motion, liquid layering and nanoparticle clustering. Moreover, You et al [9] reported that the critical heat flux in pool boiling of Al2O3 nanofluids increased dramatically (by about 200%) compared to the pure water case. Recently, Kim et al [10] studied the convective instability driven by buoyancy and the heat transfer characteristics of nanofluids.
The influences of temperature [11], particle property [12], size [13] and shape [14-15] etc on the thermal conductivity of single-species fluid have been studied by many researchers. The binary nanofluid is named after the two ingredients basefluid (ammonia and water) in solution [16]. The existence of ammonia significantly influences the dispersion stability and thermal conductivity of nanofluid, which will affect the performance of heat and mass transfer of nanofluid. However, although many studies have been conducted to study the heat transfer enhancement in single-species nanofluid, the studies on the heat transfer enhancement of multi-component nanofluids are immaterial and not systematical. Besides, the researches about the influence of adding surfactant and dispersion stability on the thermal conductivity of multi-component nanofluids are seldom found. In this work, the thermal conductivities are measured by using a KD2 Pro thermal properties analyzer. The influences of surfactant and ammonia on the dispersion stabilities of the binary nanofluids are investigated by the light absorbency ratio index methods. The influences on the thermal conductivity ratio by the type, content and size of nanoparticles, the temperature as well as the dispersion stability are experimentally studied. It is expected that this work will give some basic ideas to understand the influencing factors on the thermal conductivity of multi-components nanofluids.
2 Experimental
2.1 Preparation of binary nanofluids
The existing preparation methods of nanofluids are divided into one-step and two-step methods. One-step method is to make the nanoparticles simultaneously dispersed in the basefluid during the particle synthesis procedure, while two-step method is to add the prepared nanoparticles in their required basefluid, and then to make the nanoparticles dispersed in the basefluid by supersonic vibration, adding surfactant, or changing the pH value of basefluid etc. This work used two-step method in the preparation of ammonia-water nanofluids, which may give some basic ideas to understand the influencing factors on the thermal conductivity of industrialized nanoparticles in multi-component basefluid.
The binary nanofluids in this work are prepared by mixing Al2O3 nanoparticles with polyacrylic acid (PAA), TiO2 with polyethylene glycol (PEG) 1000, and TiN, SiC, hydroxyapatite with PEG 10000 to ammonia-water solution, respectively. The Al2O3, TiO2, SiC, TiN nano- particles are spherical or analogously spherical, while the hydroxyapatite nanoparticles are noodle like. The purities of the nanoparticles are higher than 99.5% through the detection by ultraviolet emission spectrometer. In order to investigate the influence of ammonia content on the stability and thermal conductivity of binary nanofluid, the homemade ammonia-water solution with the mass fraction of 0%, 12.5% and 25% are used as base fluid, respectively. All measurements were performed at atmospheric pressure. The existence of surfactant may have some influence on the effective thermal conductivity of nano?uids [17]. Therefore, the content of surfactant used in this work is very low so as to greatly improve the dispersion stability but has little effect on the thermal conductivity of the binary nanofluids which will be proved later in this work. According to research results of Yang et al [18] and Liu et al [19], the mass fraction of PAA and PEG 1000 used for the dispersion of Al2O3 and TiO2 nanoparticles is 0.3% and 0.25%, respectively, to obtain the better dispersion stability of the binary nanofluid. The mass faction of PEG 10000 used for the dispersion of hydroxyapatite nanoparticles in ammonia-water is 0.5% according to the dispersion research on hydroxyapatite.
2.2 Measurement of thermal conductivity
The measuring device of thermal conductivity is shown in Fig. 1. The thermal conductivity of nanofluid was measured by using a KD2 Pro thermal properties analyzer (Decagon Devices, Inc., USA). It consists of a handheld microcontroller and sensor needles. The sensor needle of KD2 contains a heating element and a thermistor. The controller module contains a battery, a 16-bit microcontroller/AD converter, and power control circuitry.
Nanofluid samples of 45 mL were taken in a vial of 30 mm in diameter whose cap is equipped with a sensor positioning device through which the sensor needle was inserted. For accurate measurements, the sensor positioning device was square-shaped whose diagonal is equal to the inside diameter of the cuvette. Thus, the sensor was inserted fully into the fluid, and oriented vertically and centrally inside the vial without touching the side walls of the vial. Insertion of the sensor needle probe into the fluid in this orientation will minimize errors from free convection [20]. In addition, the sensor needle was placed in the middle section of the nanofluid in the vial as shown in Fig. 1, so that any bubbles in the fluid would float to the top and the sedimentation would drop to the bottom away from the needle.
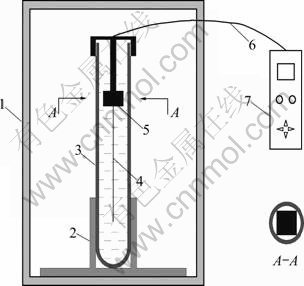
Fig. 1 Measuring device of thermal conductivity: 1- Thermotank; 2-Cuvette positioning device; 3-Cuvette 4-KD2 sensor needle; 5-Sensor positioning device; 6-Cable; 7-KD2 microcontroller
The following assumptions were made for the thermal conductivity measurement: 1) the long heat source can be treated as an infinitely long heat source; 2) the medium is both homogeneous and isotropic, and at uniform initial temperature. Although these assumptions are not true in strict sense, they are adequate for accurate thermal properties measurements. The sensor needle used was KS-1 which is made of stainless steel having a length of 60 mm and a diameter of 1.3 mm, and closely approximates the infinite line heat source which gives least disturbance to the sample during measurements. Each measurement cycle lasts for 90 s. During the first 30 s, the instrument will equilibrate which is then followed by heating and cooling of sensor needle for 30 s each. At the end of the reading, the controller computes the thermal conductivity using the change in temperature (ΔT) and time data from Ref. [20]:
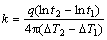 (1)
(1)
where q is constant heat rate applied to an infinitely long and small “line” source; ΔT1 and ΔT2 are the changes in the temperature at time t1 and t2, respectively.
2.3 Measurement of dispersion stability
In order to investigate the influence of dispersion stability on the thermal conductivity of binary nanofluid, the stability of nanofluids was studied by measuring the absorbency of nanofluid with and without surfactants in different mass fractions of ammonia-water basefluid after standing for 48 h. The absorbency was measured by ultraviolet-visible pectrophotometer, and the absorbency [21] of the suspension was defined by
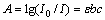 (2)
(2)
where A is absorbency; I0 is the incident light intensity; I is the transmitted light intensity; ε is molar absorptivity (l/mol·cm); b is optical path (cm); c is molar concentration (mol/L). It can be included from Eq. (1) that the absorbency is proportional to the concentration of the particles in suspension. Higher absorbency means higher concentration of nanoparticles in the solution and better dispersion of nanofluid.
3 Results and discussion
The influences of the type, content and size of nanoparticles, the temperature and the stability of nanofluid on the thermal conductivities were measured by using a KD2 Pro thermal properties analyzer. The influences of surfactant and ammonia on the dispersion stabilities of the binary nanofluids were investigated by the light absorbency ratio index methods.
The thermal conductivity ratio is defined to examine the effect of the addition of nanoparticles on the thermal conductivity of ammonia water:
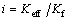 (3)
(3)
where i is the effective thermal conductivity ratio; Keff and Kf are the thermal conductivity of nanofluid and ammonia-water basefluid, respectively.
3.1 Influences of type and content of nanoparticles on thermal conductivity ratio
Figure 2 shows the experimental results of the thermal conductivity ratio of different types of nanofluid in 25% ammonia-water at 20 °C. It can be seen that the thermal conductivity ratios of all kinds of nanofluids increase approximately linearly with the increase of the content of nanoparticles. However, the rate of the increase of thermal conductivity ratio is varying and related to the type of nanoparticles. When the volume fraction of Al2O3 (40 nm) nanoparticles increases from 0.01 to 0.04, the thermal conductivity ratio increases from 1.044 to 1.176 accordingly, whose increasing rate is much higher than that of SiC nanoparticles of the same size. Similarly, the increasing rate of TiO2 (15 nm) nanoparticles is higher than that of the same sized TiN nanoparticles. Hence, the type and the content of nanoparticles are the two key influencing factors on the thermal conductivity ratio of ammonia-water nanofluids.

Fig. 2 Thermal conductivity ratio of different types of nanofluid in 25% ammonia water
3.2 Influence of size of nanoparticles on thermal conductivity ratio
The experimental results of the thermal conductivity ratio of nanofluid with different sized Al2O3 nano- particles in 25% ammonia water are shown in Fig. 3. It can be seen that the thermal conductivity ratios of nanofluid with 20 nm Al2O3 nanoparticles is higher than that with 40 nm Al2O3 nanoparticles. In the same condition, the thermal conductivity ratio of nanofluid with 40 nm hydroxylapatite nanoparticles is higher than that with 60 nm particles, as shown in Fig. 4. It seems that the thermal conductivity ratios of noodle like nanoparticles have the same variation tendency on the size dependence with the spherical nanoparticles. This experimental result is in accordance with the research results of Kim et al [22], in which the thermal conductivity ratios between nanofluid and basefluid increase nonlinearly with the decrease of the size of nanoparticles.
The possible reasons that smaller sized nanoparticles suspensions have higher thermal conductivity ratio can be explained as follows. When the size of nanoparticles is smaller, the effect of van der Waals force is stronger, and the movements of particles are more frequent and severe, the interactions between nanoparticles and between nanoparticles and liquid are stronger and the energy transmission is further, which can induce a higher thermal conductivity ratio in macroscopic view.

Fig. 3 Thermal conductivity ratio of different sized Al2O3 nanofluids in 25% ammonia-water

Fig. 4 Thermal conductivity ratio of different sized hydroxyapatite nanofluid in 25% ammonia-water
3.3 Influence of temperature on thermal conductivity ratio
Figures 5 and 6 show the experimental results of the thermal conductivity ratio of Al2O3 (40 nm) and TiO2 (20 nm) nanofluid at 20 °C and 40 °C, respectively. It can be seen that for both Al2O3 and TiO2 nanofluids, the thermal conductivity ratio increase obviously when the temperature of the nanofluid increases from 20 °C to 40 °C. The temperature dependence of thermal conductivity ratio can be explained as follows. When the temperature of the nanofluid increases, the thermal motion of nanoparticles will be enhanced. Besides, the thermal agitations of water and ammonia molecules are strengthened, and the knock-on effects [23] on the particles by the liquid molecules are stronger, which will strengthen the energy transmission and improve the thermal conductivity ratio of nanofluids.

Fig. 5 Thermal conductivity ratio of Al2O3 nanofluid at different temperatures in 25% ammonia-water

Fig. 6 Thermal conductivity ratio of TiO2 nanofluid at different temperatures in 25% ammonia-water
By comparing Fig. 3 with Fig. 5, for Al2O3 nanofluid, increasing temperature seems to be more effective than decreasing the particle size. This circumstance agrees with the experimental result of Das et al [17], in which the thermal conductivity ratios of Al2O3 nanofluid to water increase greatly when the temperature increases from 21 °C to 36 °C. It can be concluded that for ammonia-water nanofluid (binary nanofluid), the temperature dependence of thermal conductivity ratio is in accordance with that of single-species nanofluid.
3.4 Influence of stability on thermal conductivity ratio
In order to investigate the influence of dispersion stability on the thermal conductivity ratio of binary nanofluid, the comparative experiments on the thermal conductivity ratio of the binary nanofluid with and without surfactant, and nanofluid with different contents of ammonia were carried out.
The comparative experiment results on the thermal conductivity ratio of the Al2O3 nanofluid with 0.25% PAA and without surfactant, TiO2 nanofluid with 0.3% PEG 1000 and without surfactant are shown in Fig. 7. It can be seen that, especially for Al2O3 nanofluid, the thermal conductivity ratio of the nanofluid with surfactant is obviously higher than that without surfactant. The surfactants have little influences on the thermal conductivity ratio, which will be proved later in this work. The reason of surfactants performing positive roles in the thermal conductivities of binary nanofluid can be attributed to the improvements of surfactants to the dispersion stabilities of nanofluid, as shown in Fig. 8. It can be seen that after standing for 48 h, the absorbency of nanofluid with surfactant is much higher than that without surfactant. Especially for Al2O3 nanofluid, the absorbency approaches to zero, which means that almost all the Al2O3 nanoparticles drop on the bottom of the test tube after 2 d static storage. This is the reason that the thermal conductivity ratio will decrease greatly without surfactant.

Fig. 7 Thermal conductivity ratio of binary nanofluids with and without surfactant
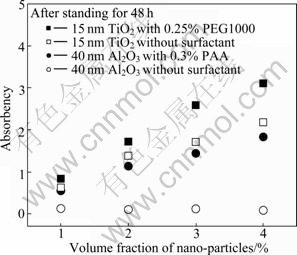
Fig. 8 Absorbency of binary nanofluids with and without after standing for 48 h
The nanoparticles have high specific surface area and intense surface activity, which directly results in colliding and reuniting among the nanoparticles. Therefore, it is difficult for them to be dispersed steadily and uniformly in the ammonia-water solution without surfactant. The effect of surfactants on improving the dispersion can be explained as follows. An interfacial film surrounding nanoparticles will be formed as a result of the absorption of dispersant on the interface. When the intensity and thickness of interfacial film are enough, it can protect the nanoparticles from aggregating [24]. The poor stabilized nanoparticles will collide and drop on the bottom of the cuvette, and they cannot make contributions to the thermal conductivity of fluid. Even if the poor stabilized nanoparticles suspend in the fluid temporarily, the particles are reunited to large sized particles (micrometer-size or even larger), thus the relatively large reunion cannot achieve some superior properties of the nanoparticles, such as the micro- convection and high specific surface area. The enhancement of thermal conductivity of nanoparticles cannot be fully functioned in the nanofluid of poor stability. So the thermal conductivity of poorly stabilized nanofluid is much less than that of stable nanofluid with adding proper surfactant.
The influence of ammonia content on the thermal conductivity ratio of binary nanofluid is also studied and the thermal conductivity ratio of Al2O3, TiO2, SiC and TiN nanoparticles with the volume fraction of 4% in 0%, 12.5%, 25% ammonia-water are shown in Fig. 9. It can be found that the thermal conductivity ratio of ammonia- water basefluid decreases with the rising of ammonia content. However, the thermal conductivity ratios of the two kinds of Al2O3, TiO2 and SiC binary nanofluid with ammonia-water as basefluid are much larger than those with distilled water as basefluid, which means that increasing the ammonia content can improve the thermal conductivity ratios of the three kinds of nanofluid. However, for TiN nanofluid, the thermal conductivity ratio increases firstly, and then sharply decreases with the increase of ammonia content. The influence of ammonia content on the thermal conductivity ratio of binary nanofluid can be attributed to the influence of ammonia content on the dispersion stability, as shown in Fig.10.
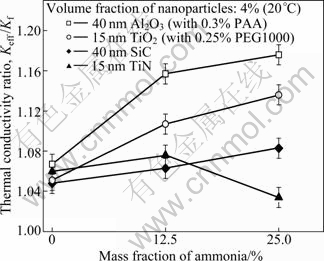
Fig. 9 Thermal conductivity ratio of binary nanofluids with different contents of ammonia
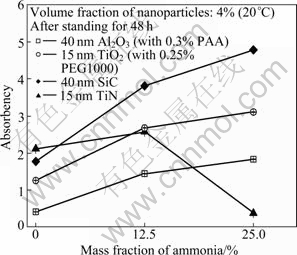
Fig. 10 Absorbency of binary nanofluid with different contents of ammonia after standing for 48 h
Figure 10 shows the absorbencies of the binary nanofluids with the ammonia content after standing for 48 h. It can be seen that the absorbency of Al2O3, TiO2 and SiC nano?uid with distilled water (DW) was far less than that of nano?uids with ammonia-water and the absorbency will increase with the increase of the mass fraction of ammonia-water base?uid. Suspension stability is directly dependent on the Zeta potential of the powder that represents the potential difference between the surface of the grains and the external plane of Helmholtz. The Zeta potential is thus defined as the electrical potential developed at the solid/liquid interface in response to the relative movement of solid particles and liquid or as the strength of the particle electrical barrier. The higher the potential with the same polarity is, the higher the electrostatic repulsion between particles becomes. On the other hand, when the suspension is close to the iso-electric point, the particles tend to flocculate. The Zeta potential of nanoparticles is related to the pH value of the base?uid. The dispersion stability of Al2O3 and TiO2 nano?uids with distilled water poorer than that in ammonia water can be explained as follows: According to Refs. [19, 25-26], the pH values of iso-electric points of Al2O3, TiO2 and SiC are below or close to 7. With the increases of mass fractions of ammonia-water base solution, the pH value of solutions increases, the Zeta potential of nano?uids is far away from iso-electric point and higher, and the high Zeta potential of nanofluids can keep a high electrostatic repulsion which can keep the dispersion more stable and uniform. However, for TiN nanofluid, according to Ref. [27], although the pH value of iso-electric point is below 7, the Zeta potential increases firstly and then decreases with the increase of pH value of basefluid, which induces a poor dispersion stability of TiN nanofluid in too higher pH value fluid. So, the dispersion stability of TiN nanofluid is improved firstly and then deteriorated with the increase of pH value related to the content of ammonia.
The dispersion stabilities of nanofluids are greatly improved by obtaining proper ammonia contents due to the high Zeta potentials. Thus, the thermal conductivity enhancement of the nanofluid can be fully functioned in a uniformly dispersion condition.
3.5 Influence of surfactant on thermal conductivity
Figure 11 shows the influences of surfactants on the thermal conductivity of basefluid. It can be seen that the three kinds of surfactants in low concentration have relatively smaller influence than other influence factors on thermal conductivity, like, particles or ammonia content, and temperature, because the maximum deviation of the surfactant on the thermal conductivity of basefluid is 1.44%. Additionally, by comparing Fig. 11 to other figures on thermal conductivity of nanofluid in this work, it can be found that the fluctuation ranges of the measured value of thermal conductivity of ammonia- water or ammonia-water with surfactants are much smaller than those with nanoparticles. This may be caused by the chaotic collision of nanoparticles on the sensor needle of KD2 Pro thermal properties analyzer.
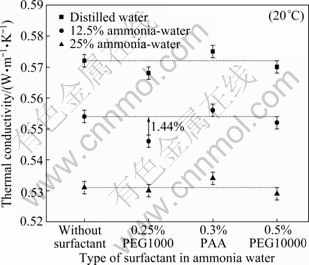
Fig. 11 Thermal conductivity of ammonia-water base fluid with different kinds of surfactant
4 Conclusions
1) The type, content and size of nanoparticles, the temperature as well as the dispersion stability are the key parameters that affect the thermal conductivity of nanofluids.
2) For the given nanoparticle material and the base fluid, the thermal conductivity ratio of the nanofluid to the ammonia-water liquid increases as the nanoparticle content and the temperature are increased, and the nanoparticle diameter is decreased.
3) The thermal conductivity ratios increase significantly by improving the stabilities of nanofluids, which is achieved by adding surfactants or performing the proper ammonia content in the fluid.
4) Adding surfactants can improve the stability and the thermal conductivity of the binary nanofluid, but the surfactants in low concentrations have relatively smaller influence on the thermal conductivity.
References
[1] KANG Y T, KIM J, KIM J K. Comparisons of mechanical, chemical and nano technologies for absorption applications [C]// Proceedings of International Seminar on Thermally Powered Sorption Technology. Fukuoka, Japan, 2003: 69-77.
[2] XU Jie, YU Bo-ming, ZOU Ming-qing, XU Peng. A new model for heat conduction of nanofluids based on fractal distributions of nanoparticles [J]. Journal of Physics D: Applied Physics, 2006, 39(20): 4486-4490.
[3] Eiyad A N, Ziyad M, Hakan F. Effect of nanofluid variable properties on natural convection in enclosures [J]. International Journal of Thermal Sciences, 2010, 49(3): 479-491.
[4] Yang Bao, Han Zeng-hu. Thermal conductivity enhancement in water-in-FC72 nanoemulsion fluids [J]. Applied Physics Letters, 2006, 88(26): 261914.
[5] LEE J K, KOO J, HONG H, KANG Y T. The effects of nanoparticles on absorption heat and mass transfer performance in NH3/H2O binary nanofluids [J]. International Journal of Refrigeration, 2010, 33(2): 269-275.
[6] Yang Liu, Du Kai, Zhang Xiao-song, Cheng Bo. Experimental study on enhancement of ammonia-water falling film absorption by adding nano-particles [J]. International Journal of Refrigeration, 2011, 34(3): 640-647.
[7] CHOI S U S. Enhancing thermal conductivity of fluids with nano-particles [M]// Siginer D A, Wang H P. Development and Applications of Non-Newtonian Flows, EFD. New York: ASME, 1995: 99-105
[8] KeblinskI P, Pillpot S R, Choi S U S, Eastman J A. Mechanism of heat flow in suspensions of nano-sized particles (nanofluids) [J]. Int J Heat Mass Transfer, 2002, 45(4): 855-863.
[9] You S M, Kim J H, KiM K H. Effect of nano-particles on critical heat flux of water in pool boiling heat transfer [J]. Appl Phys Lett, 2003, 83(16): 3374-3376,
[10] Kim J, Kang Y T, Choi C K. Analysis of convective instability and heat transfer characteristics of nanofluids [J]. Phys Fluids, 2004, 16(7): 2395-2401.
[11] Li C H, Peterson G P. Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticles suspensions (nanofluids) [J]. Journal of Applied Physics, 2006, 99(8): 084314.
[12] Wang Xin-wei, Xu Xian-fan, Choi S U S. Thermal conductivity of nanoparticles-fluid mixture [J]. Journal of Thermophysics and Heat Transfer, 1999, 13(4): 474-480.
[13] Anoop K B, Sundararajan T, Das S K. Effect of particle size on the convective heat transfer in nanofluid in the developing region [J]. International Journal of Heat and Mass Transfer, 2009, 52(9/10): 2189-2195.
[14] Xie Hua-qing, Wang Jin-chang, Xi Tong-geng, Liu Yan. Thermal conductivity of suspensions containing nanosized SiC particles [J]. International Journal of Thermophysics, 2002, 23(2): 571-580.
[15] Murshed S M S, Leong K C, Yang C, Enhanced thermal conductivity of TiO2-water based nanofluids [J]. International Journal of Thermal Science, 2005, 44(4): 367-373.
[16] Su Feng-Min, Ma Xue-hu, Chen Jia-bin, Zhang Yong. Study of thermal conductivity of binary nanofluids [J]. Journal of Dalian University of Technology, 2008, 48(6): 782-785. (in Chinese)
[17] Das S K, Putra N, Thiesen P, Roetzel W. Temperature dependence of thermal conductivity enhancement for nano?uids [J]. J Heat Transfer, 2003, 125(4): 567-574.
[18] Yang Liu, Du Kai, Zhang Xiao-song, Cheng Bo. Preparation and stability of Al2O3 nano-particle [J]. Applied Thermal Engineering, 2011, 31(17/18): 3643-3647.
[19] Liu Rui-hua, Dong Yong-chun, Wang Xiao-ni, Yan Hai-li. The preparation and stability of TiO2 nanofluid [C]// The Fifth Processing of Functional Textiles and Nanotechnology. China, 2005: 310-313.
[20] Chandrasekar M, Suresh S, Chandra Bose A. Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid [J]. Experimental Thermal and Fluid Science, 2010, 34(2): 210-216.
[21] Li Chang-hou. Ultraviolet-visible pectrophotometer [M]. Beijing: Beijing Chemical Industry Press, 2005: 8-11. (in Chinese)
[22] Kim S H, Choi S R, Kim D. Thermal conductivity of metal-oxide nanofluids: Particles size dependence and effect of laser irradiation [J]. ASME Journal of Heat Transfer, 2007, 129(3): 298-307.
[23] Xuan Yi-min, Li Qiang. Energy transfer theory and application of nanofluid [M]. Beijing: Science Press, 2010: 86-88. (in Chinese)
[24] Li Feng-sheng, Cui Ping, Yang Yi. The treatment technology and application of micrometer-nanometer powders [M]. Beijing: Beijing National Defense Industrial Press, 2005: 27-33. (in Chinese)
[25] Sun Yu-li, Zuo Dun-wen, Zhu Yong-wei. Dispersing of α-Al2O3 nano-powers in water suspension [J]. Journal of Nanjing University of Aeronautics and Astronautics, 2008, 40(5): 702-706. (in Chinese)
[26] Hou Yao-yong, Li Li, Yang Jing-yi, YANG Feng-ke. Preparation of highly dispersed and stabilized α-Al2O3 and nano-SiC single-phase and mixed aqueous suspension [J]. Journal of the Chinese Ceramic Society, 1998, 26(2): 171-177. (in Chinese)
[27] Feng Ping, Xiong Wei-hao. Dispersion behavior of nanosized TiN powder in water and absolute ethyl alcohol [J]. The Chinese Journal of Process Engineering, 2005, 5(1): 62-65. (in Chinese)
(Edited by HE Yun-bin)
Foundation item: Projects(51176029, 50876020) supported by the National Natural Science Foundation of China; Project(2011BAJ03B00) supported by the 12th Five-Year National Science and Technology Support Key Program of China; Project(ybjj1124) supported by the Foundation of Graduate School of Southeast University, China
Received date: 2011-07-26; Accepted date: 2011-11-14
Corresponding author: DU Kai, Professor; Tel: +86-25-83792314; E-mail: du-kai@seu.edu.cn