Self-assembled activated carbon sandwiched graphene film for symmetrical supercapacitors
来源期刊:中南大学学报(英文版)2020年第12期
论文作者:朱连文 谷俐 陆绍荣 武圆圆 陈汝婷 曹雪波
文章页码:3603 - 3614
Key words:graphene film; activated carbon; self-assembly; supercapacitor
Abstract: Activated carbon (AC) particles sandwiched reduced graphene oxide sheets (rGO) film has been successfully fabricated via a facile self-assemble approach. The as-formed AC/rGO film is self-standing, flexible and mechanically robust, allowing to be transferred to any substrate on demand without rupture. Since AC particles effectively suppressed the restacking of the rGO sheet, AC/rGO film exhibits loose layer-by-layer stacking structures with various gaps between AC particles and rGO sheets, which is different from compact structures of pure graphene films. The as-formed gaps provide fast diffusion channels for electrolyte ions and enhanced accessible surface area of rGO. Therefore, the AC/rGO electrode delivers improved electrochemical performance over the voltage range of 0.0-3.0 V. This work offers a promising strategy to design free-standing supercapacitor electrodes based on traditional nanocarbon materials.
Cite this article as: WU Yuan-yuan, ZHU Lian-wen, CHEN Ru-ting, GU Li, CAO Xue-bo, LU Shao-rong. Self-assembled activated carbon sandwiched graphene film for symmetrical supercapacitors [J]. Journal of Central South University, 2020, 27(12): 3603-3614. DOI: https://doi.org/10.1007/s11771-020-4505-9.

J. Cent. South Univ. (2020) 27: 3603-3614
DOI: https://doi.org/10.1007/s11771-020-4505-9

WU Yuan-yuan(武圆圆)1, 2, ZHU Lian-wen(朱连文)3, CHEN Ru-ting(陈汝婷)4, GU Li(谷俐)1, 2, CAO Xue-bo(曹雪波)3, LU Shao-rong(陆绍荣)1
1. College of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, China;
2. School of Materials and Textile Engineering, Jiaxing University, Jiaxing 314001, China;
3. College of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, China;
4. Department of Materials, Imperial College London, London, SW72AZ, United Kingdom
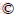 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Activated carbon (AC) particles sandwiched reduced graphene oxide sheets (rGO) film has been successfully fabricated via a facile self-assemble approach. The as-formed AC/rGO film is self-standing, flexible and mechanically robust, allowing to be transferred to any substrate on demand without rupture. Since AC particles effectively suppressed the restacking of the rGO sheet, AC/rGO film exhibits loose layer-by-layer stacking structures with various gaps between AC particles and rGO sheets, which is different from compact structures of pure graphene films. The as-formed gaps provide fast diffusion channels for electrolyte ions and enhanced accessible surface area of rGO. Therefore, the AC/rGO electrode delivers improved electrochemical performance over the voltage range of 0.0-3.0 V. This work offers a promising strategy to design free-standing supercapacitor electrodes based on traditional nanocarbon materials.
Key words: graphene film; activated carbon; self-assembly; supercapacitor
Cite this article as: WU Yuan-yuan, ZHU Lian-wen, CHEN Ru-ting, GU Li, CAO Xue-bo, LU Shao-rong. Self-assembled activated carbon sandwiched graphene film for symmetrical supercapacitors [J]. Journal of Central South University, 2020, 27(12): 3603-3614. DOI: https://doi.org/10.1007/s11771-020-4505-9.
1 Introduction
Along with the rapid growth of market demands for wearable and portable electronics, including smart cloths, roll-up displays, electronic sensors, there is an urgent need for developing equally flexible energy storage devices, such as flexible supercapacitors and batteries [1-3]. Unlike batteries, which chemically store electric energy, supercapacitors, particularly electrochemical double layer capacitors (EDLCs), which store and release energy through physical processes, in which ions are reversibly adsorbed-desorbed at the electrode/ electrolyte interface [4-8], this fast charging/ discharging process don’t involve phase and composition changes, enabling EDLCs with unrivaled durability (>100000 cycles), excellent safety, wide range of operating temperature, and high-power-density [7-9]. However, the assembly of traditional supercapacitor devices often involves the use of current collector, binder and conductive additive, which dramatically drag down the specific energy density [10, 11].
Therefore, it is highly demanded to design flexible electrode materials without current collector, binder and conductive additive for flexible EDLCs to power wearable electronic devices.
Graphene is a promising electrode material for supercapacitors due to its many unique properties, including a huge theoretical specific surface area (2300 m2/g), high electrical conductivity, excellent stability and high theoretical capacitance (~550 F/g) [12-15]. Especially, graphene sheets could be easily organized into highly flexible and mechanically robust films driven by the strong π-π interaction between adjacent layers [16, 17]. Therefore, graphene films hold great promise as flexible supercapacitor electrodes. Unfortunately, the tightly stacked graphene sheets hinder the electrolytes diffusion into the gaps of graphene films to form electrical double layers, which leads to a dramatically diminished electrochemically active specific surface area and specific capacitances [18, 19]. To avoid this issue, one effective approach is intercalating some spacers between graphene sheets to form composite films, such as carbon nanotubes (CNTs) [20], nanodiamond [21], polymers [22], and metal oxide [23]. The intercalated spacers would create efficient pathways for electrolyte ions diffusion and enhance the accessible surface area, while still maintain the mechanical strength of graphene films, thus achieving improved supercapacitor performance.
Nowadays, many methods, including suction filtration [20], layer-by-layer (LBL) self-assembly [22], and solution casting [24], have been applied to sandwich spacers between graphene sheets. YU et al[25] demonstrated the first CNT intercalated rGO films by LBL electrostatic assembly process. The resulting composite films delivered a nearly rectangular cyclic voltammogram curve and increased specific capacitance of 120 F/g. LI et al [26] have reported activated carbon and CNT sandwiched rGO film via vacuum filtration. The SCs based on resulting films exhibited a specific capacitance of 101 F/g. Comparing with other film preparation methods, the self-assemble route is simple, scalable, and applicable to all kinds of nanostructures [16, 27, 28], which allows tuning the film composition, thickness, size and microstructure to meet the demands of applications. However, as far as we know, direct growth of carbon intercalated graphene film for energy storage via a self-assembly process is still a big challenge.
Our previous research demonstrates that copper can induce various carbon nanostructures, such as graphene oxide, CNTs, carbon dots and carbon nanoparticles, to spontaneously grow into large area, ordered films under mild conditions [16, 27, 28]. This active methodology enables precise and compact alignment of the carbon units, achieving films with well-ordered structure and high-uniformity over films fabricated by other methods [16, 27, 28]. Thus, we are inspired to speculate that the copper-induced growth process might be a promising method to organize two kinds of nanostructured carbons into large-area composite films, leading to the in-situ formation of carbon intercalated carbon films. Herein, GO and activated carbon (AC) were employed as carbon units to demonstrate this concept. By immersing copper into the homogeneous AC and GO mixture dispersion, large area AC sandwiched graphene composite films have been fabricated. The films deliver high flexibility, excellent mechanical strength, high uniformity, and good electrical conductivity. The intercalated AC creates efficient pathways for electrolyte ions diffusion and increases the accessible surface area, thus achieving higher specific capacitances and energy density.
2 Experimental
2.1 Materials
All the reagents were commercially available and used as-received, except for the GO. The GO was prepared through the modified Hummers method [27]. 1 g of flake graphite, 0.75 g of NaNO3 and 34 mL of H2SO4 (~98%) were placed in a single neck flask and stirred under ice-cooling. 5 g of KMnO4 was divided into 5 to 10 times and added with stirring at 40 °C. About 2 h later, 50 mL of deionized water was slowly added. After waiting for cooling, 4 mL 30% hydrogen peroxide was added one time. Finally, it was repeatedly washed with deionized water until the supernatant was neutral and dispersed in 1 L of deionized water. 20 mL of graphene oxide solution, 180 mL of deionized water and 0.2 g of activated carbon without any treatment were mixed. Use 2% Triton-100 to promote the dispersion of activated carbon. The solution was then adjusted to pH=4 with oxalic acid and sonicated for 30 min before use.
2.2 Manufacture of AC/rGO films
To assemble the AC/rGO, a petri dish was filled with 30 mL of the AC/GO dispersion and then a piece of battery-grade copper foil was placed on the solution surface. The film growth process was conducted at 45 °C. After 6 h, the surface of copper foil in contact with the solution turned black, indicating the successful formation of black AC/rGO layer. Extending the growth time would increase the film thickness. The growth time was set to 24 h in this experiment. After the growth of the AC/rGO film with a desirable thickness, the copper foil was taken out, carefully rinsed with deionized water, and dried in air. The assembly of GO film was carried out with GO solution under the same reaction conditions as AC/rGO film. To obtain free-standing AC/rGO film without copper foil support, the copper foil underlying the film has been dissolved by 0.1 mol/L K2S2O8 solution. After etching, the isolated AC/GO film would float freely on the solution surface and can be transferred to substrates on demand without rupturing. Then, AC/rGO film was washed twice with ultra-pure water and dried in air. Finally, the film was reduced at 400 °C for 2 h in H2/Ar atmosphere.
2.3 Characterization
The microstructures of the carbon films were observed by scanning electron microscopy (SEM Hitachi S4800). A Bruker D8 focus powder X-ray diffractometer using Cu-Kα radiation was used to record XRD data. Raman spectrum was obtained from a LabRAM HR800 Raman spectrometer with a 532 nm wavelength incident laser. Thermo Nicolet AVATAR 320 instrument was applied to record Fourier transform infrared spectrum. Nitrogen adsorption–desorption isotherms were obtained on a micromeritics ASAP 2020 instrument. X-ray photoelectron spectra (XPS) were obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300 W Al Ka radiation.
2.4 Assembly and characterization of symmetrical supercapacitors
Coin-type EDLC cells with two symmetrical carbon electrodes were assembled using the prepared carbon materials.
EDLC cells with two symmetrical carbon electrodes were assembled with a CR2032 size using the prepared AC/rGO film and glass microfiber filter. 300 μL of organic electrolyte (1 mol/L of Et4NBF4 in PC) was injected and the supercapacitor was left overnight until the electrolyte diffused completely. The cell was then sealed using a coin cell crimper. All steps for assembling the EDLC cell were carried out in an inert gas filled glove box filled with water and oxygen content both less than 0.1×10-6. The electrochemical performance of the cells was studied by cyclic voltammetry (CV), electrochemical impedance spectroscopy and galvanostatic charge/discharge (C/D) using a CHI 760C work station at room temperature. CV measurement was carried out in the voltage range of 0-3 V at different scan rates ranging from 10 to 300 mV/s. The specific capacitance of prepared carbon films was calculated using the following well- known equation:

where C is the specific capacitance; I is the loaded current; m is the mass of carbon film; △t is the discharge time; △V is the potential change during the discharge process.
3 Results and discussion
Figure 1 exhibits the direct growth process of AC/rGO films. In a typical experiment, AC particles were homogenously dispersed in GO solution by vigorous ultrasonication and then a piece of Cu foil was immersed in the dispersion. Black and dense film would be formed on copper foil after overnight reaction due to the spontaneous deposition of AC particles and GO sheets on copper. According to our previous report [16, 27, 28], electrical double layer theory was applied to explain the direct self-assembly of activated carbon and graphene sheets on copper foil to form AC/rGO composite films. When the copper foil was immersed into homogeneous AC and GO mixture dispersion, the copper foil would release Cu2+ ions into the mixture dispersion driven by the attraction of polar H2O molecules. Therefore, an electrical double layer is formed at the copper/dispersion interface. GO sheets and AC particles exhibit negative charges due to the abundant surface oxygen-containing groups, which has been proved by FT-IR shown. Therefore, GO sheets and AC particles can be electrically anchored onto the surface of the Cu foil. Owing to the intimate contact of carbon film with Cu foil, electrons are transferred from copper to AC/GO film, leading to the reduction of the GO sheets and formation of AC sandwiched rGO film. It is necessary to emphasize that the electrical double layer theory can also be used to explain the synthesis mechanism of graphene based composite materials, such as Sb@Sb2O3/rGO composite and Ag/graphene composite [29, 30]. By repeating the above adsorption and reduction process, large-area AC/rGO films with desirable thickness are eventually formed (Figure 1). Alternatively, isolated AC/rGO films were obtained by chemically etching the copper substrate with 0.1 mol/L K2S2O8 solution. Similarly, the pure rGO film has also been prepared via the same method.
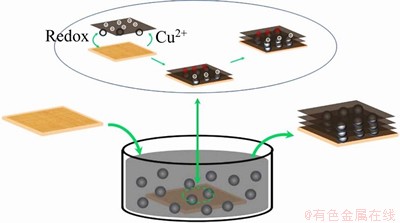
Figure 1 Schematically illustrating direct growth process of AC/rGO films
Figures 2(a) and 2(b) exhibit the photographs of the self-assembled AC/rGO composite film and rGO film. Both films are self-standing, flexible and mechanically robust, allowing to be transferred to any substrate on demand without rupture. Figures 2(c) and 2(d) show the top surface SEM images of AC/rGO composited film and rGO film. The AC/rGO composite film exhibits smooth surface with a few ripples and no AC particles can be seen on the film surface, which is highly similar to pure rGO film (Figure 2(d)), indicating that the surface layer of the film mainly consists of stacked graphene sheets. As shown in Figure 2(e), it can be clearly seen that the AC/rGO films deliver loose layer-by-layer stacking structures with intercalated AC particles (Indicated by blue arrows) between graphene sheets, which is different from compact layer-by-layer stacking structures of pure graphene films (Figure 2(f)). For pure rGO film, rGO sheets are tightly stacked layer by layer driven by strong van der Waals force and hydrogen bonding [16], resulting in highly compacted and oriented layer structure, which is unfavorable for electrolytes diffusion and electrical double layers formation. Therefore, the specific capacitance of graphene film is far below the theoretical capacitance of graphene (~550 F/g). For AC/rGO film, AC particles sandwiched between stacked graphene sheets, resulting in the formation of various gaps with size ranging from several tens of nanometers to several hundreds of nanometers, which is consistent with the size of AC particles (Figure 3). The as-formed gaps not only serve as fast diffusion channels for electrolyte ions, but also increase accessible surface area of rGO, thus achieving higher specific capacitances and energy density than pure rGO film.
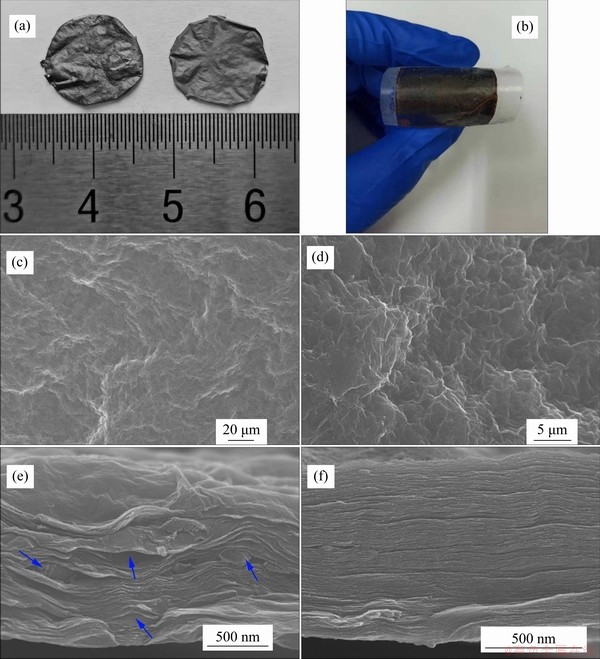
Figure 2 (a) Photographs of AC/rGO composited film (left) and pure rGO film (right); (b) AC/rGO film transferred to substrate; SEM imagines taken on surface (c) and cross section (e) of AC/rGO film (AC particles were indicated by blue arrows); SEM imagines taken on surface (d) and cross-section (f) of pure rGO film
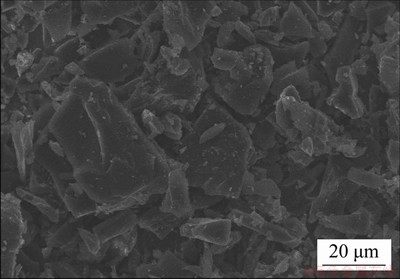
Figure 3 SEM image of AC particles
Figure 4(a) exhibits the XRD patterns of AC/rGO composite film, pure rGO film and AC particles. Pure rGO film delivers a very strong diffraction peak around 2θ of 25.6o, which is corresponding to the (002) plane of rGO [31, 32]. The AC shows two weak peaks centered at 23.5o and 43.2o, indicating its poor long-range ordering nature. For AC/rGO film, the XRD pattern shows the combined characteristic features of AC particles and rGO sheets, and the peak located at 43.2o ascribed to AC is not obvious mainly because of its low content in the AC/rGO composite film. Figure 4(b) presents the FT-IR spectra of AC/rGO composite film, pure rGO film, and AC particles. Both pure rGO film and AC show absorption peaks around 3425, 1650, 1386 and 1105 cm-1, resulting from the stretching vibration of oxygen-containing groups, such as —OH, C=C, —COOH and C—O—C [26], respectively. The oxygen-containing groups (such as —OH and —COOH) on GO and AC would oxidize copper foil, leading to the spontaneous deposition of AC and GO on copper and the formation of black and dense film coating on copper foil after overnight reaction [16, 27, 28]. AC/rGO film delivers all the absorption peaks originated from AC and pure rGO film with some slight shifts, which indicates the existence of π-π interaction between AC and rGO sheets. Figure 4(c) shows the Raman spectra of the AC/rGO composite film, pure rGO film, and AC particles. All the products deliver similar D band at wavenumbers ~1350 and G band at wavenumbers ~1590 cm-1. It is well recognized that G band is originated from the vibration mode of sp2-bonded carbon atoms, while D band is attributed to the disordered sp3 carbon atoms. The relative intensity ratio of ID/IG is indicative of the lattice defect and structural disorder degree in carbon materials [33]. Among all the samples, AC particles exhibit the smallest ID/IG value (0.74), which is in good agreement of their less defective and structurally disordered feature. The highest ID/IG ratio (0.97) of rGO film reveals the existence of lattice defects and disordered structures on the rGO sheet, which is originated from the metal-induced reduction process and residual oxygen-containing functional groups [34]. The ID/IG ratio of AC/rGO composite film (0.91) is between pure rGO film and AC, indicating the successful formation of composite film via copper-induced self-assemble process. D+G band centered around 2950 cm-1 is often applied to confirm the graphene layer number [26, 33]. Compared with pure rGO film, AC/rGO delivers obvious D+G band, which demonstrates that the sandwiched AC particles could hinder the restacking of the rGO sheet during the direct growth process.
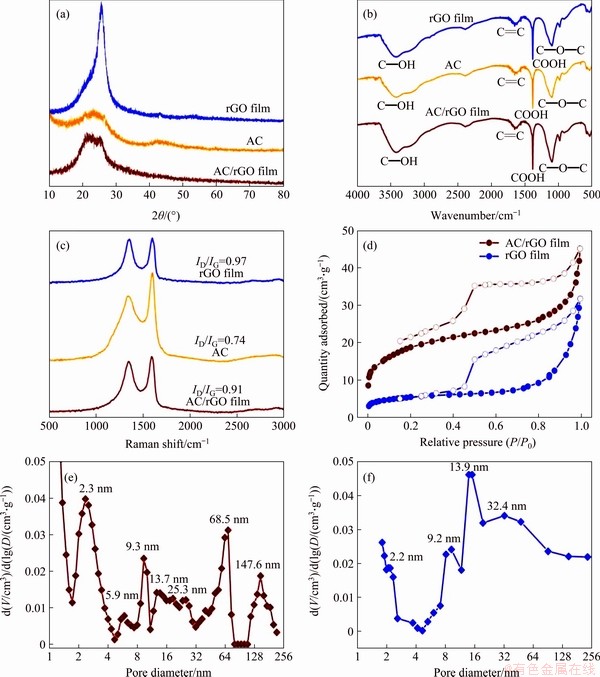
Figure 4 XRD patterns (a), FT-IR spectra (b) and Raman spectra (c) of self-assembled AC/rGO composite film, pure rGO film and AC particles; (d) Nitrogen adsorption-desorption isotherms of AC/rGO composite film and pure rGO film (Solid symbol represents adsorption, while hollow symbol denotes desorption); Pore-size distribution of AC/rGO composite film (e) and pure rGO film (f)
BET surface areas of the samples were measured by the N2 adsorption-desorption measurement (Figures 4(d)-(f) and Figure 5). The corresponding specific surface areas of rGO film, AC particles and AC/rGO composite film are 19.5, 1503.5, and 68.1 m2/g, respectively. The specific BET surface area of AC/rGO is three times higher than the pure rGO film (19.5 m2/g), indicating that the sandwiched AC particles effectively suppressed the restacking of the rGO, which enables AC/rGO to exhibit improved electrochemical performance [26]. Figures 3(e) and (f) show the pore size distributions of AC/rGO composite film and rGO film. Pure rGO film only shows mesopore size ranging from 2 nm to 32 nm, while AC/rGO film exhibits bimodal pore structures including mesopores ranging from 2 nm to 32 nm and macropores around 68.5 nm and 147.6 nm. Comparing with rGO film, the enhanced mesopores of AC/rGO film are ascribed to the sandwiched AC particles (Figure 5). The new emerging macropores of AC/rGO film are originating from the gap between sandwiched AC particles and graphene sheets, which is consistent with the cross-section SEM imagine.
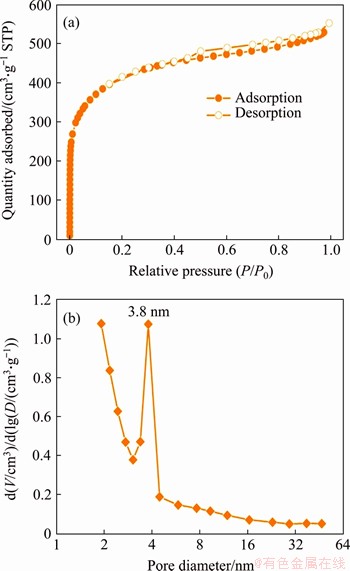
Figure 5 N2 adsorption/desorption isotherms (a) and pore size distribution (b) of AC particles
The electrochemical performance of the self-assembled films was investigated by electrochemical impedance spectroscopy (EIS) measurement. Figure 6 exhibits the impedance spectra of AC particles, rGO film and AC/rGO composite film. The Nyquist plots of all products exhibit a semicircle at high-frequency and an oblique line at low-frequency. Small semicircle at high-frequency represents low charge transfer resistance, which is beneficial for energy storage application. Figure 6(a) reveals that the AC particles show the highest charge transfer resistance due to their poor electronic conductivity, which is good consistent with previous papers. Figure 6(b) suggests that rGO film exhibits the smallest charge transfer resistance, which might be ascribed to the compact stacking structure and high reduction degree by copper foil during the self-assemble process. The charge transfer resistance of AC/rGO film is much smaller than that of AC particles, but slightly bigger than pure rGO film. For AC/rGO film, the sandwiched AC particles create various gaps and loose layer-by-layer structure, which would increase ion transport pathway and decrease the contact area between adjacent rGO sheets,resulting in a fast ion transport and relatively slow electron transport compared to rGO film. Therefore, AC/rGO films keep good balance between ion transport and electron transport, which will be favorable for excellent electrochemical performance.
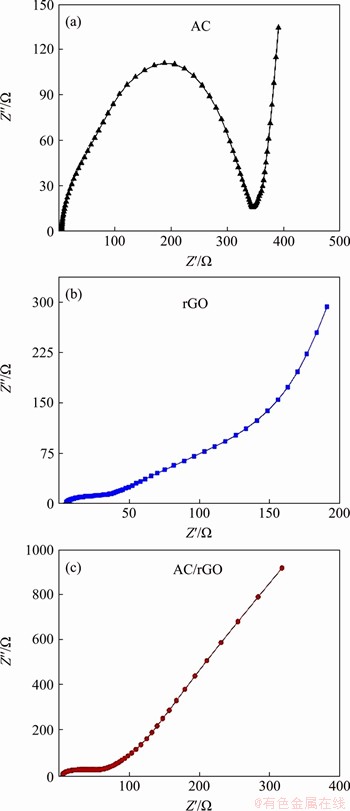
Figure 6 Impedance spectra of AC (a), rGO film (b) and AC/rGO film (c)
Figure 7(a) shows the CV curves of the assembled EDLC cells using the AC/rGO composite film electrode at various scan rate of 10, 20, 50, 100, 200 and 300 mV/s in the voltage range of 0-3.0 V. AC/rGO film presents slightly deformed rectangle-shaped CV curves at various scan rate, suggesting the EDLC energy storage mechanism and good power capability of the assembled device. Comparing to pure rGO, AC/rGO film electrode exhibits enhanced CV area (Figure 8) and increased specific capacitance by 15%, which reveals the key roles of sandwiched AC particles in electrochemical performance. The poor performance of pure rGO film electrode is due to the serious restacking layer structures, which hinder electrolytes diffusion and electrical double layers formation. For AC/rGO film, the intercalated AC particles prevent the severe restacking of the rGO sheet, which effectively enhances the electrochemically active surface area and creates efficient pathways for electrolyte ions diffusion, while maintaining the low charge transfer resistance, thus achieving larger CV area and higher specific capacitance. Figure 7(b) exhibits the galvanostatic charge/discharge (GCD) measurements of the assembled double layer supercapacitors. All GCD curves present exhibited a typical isosceles triangular shape, indicating small mass transfer resistance and excellent charge propagation behavior of the electrolyte ions in the porous AC/rGO composite film electrode [35]. Figures 7(c) and 7(d) exhibits the long-term cycling performance of AC/rGO composite film electrode in the voltage range of 0-3.0 V with the current density of 10 A/g. The AC/rGO electrode possesses an initial discharge specific capacitance of 33.6 F/g (~15% higher than pure rGO), and exhibits a low capacitance loss of ~0.02% per 100 cycles (94% capacitance retention after 30000 cycles). The excellent stability and long-term cycling performance of AC/rGO device are attributed to the unique and stable AC sandwiched graphene sheets structure of the electrode. The XPS spectrum (Figure 9) reveals the unchanged carbon skeleton and slight variation of oxygen-containing functional groups of the AC/rGO film after 30000-cycle operations, demonstrating the high stability and reliability of the electrode. For two devices in series, they can light up sixteen green light emitting diodes (Inset in Figure 7(d)). LEDs driven by supercapacitors are very common in previous reports, but it is rarely to see such small coin-shaped supercapacitors can power 16 LEDs, thereby demonstrating the high energy density and power density of the assembled AC/rGO devices.
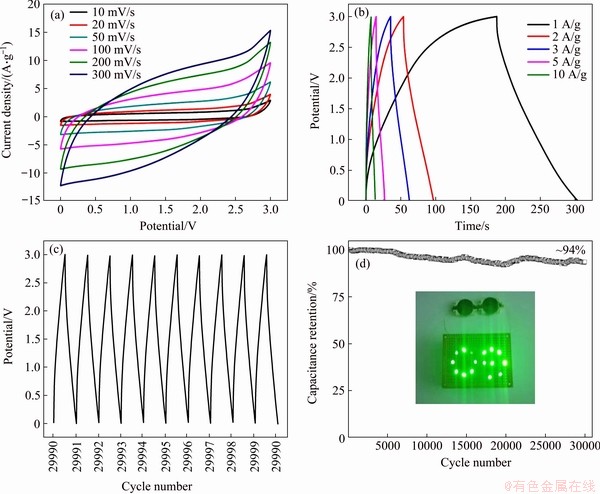
Figure 7 (a) CV curves of AC/rGO composite film electrode at various scan rates of 10, 20, 50, 100, 200 and 300 mV/s, respectively; (b) Charge and discharge curves of AC/rGO film electrode; (c) Galvanostatic charge/discharge curves for the last ten cycles (29990-30000); (d) Long-term cycling performance of AC/rGO composite film electrode. (Inset shows that two devices can light up sixteen green LEDs in parallel)
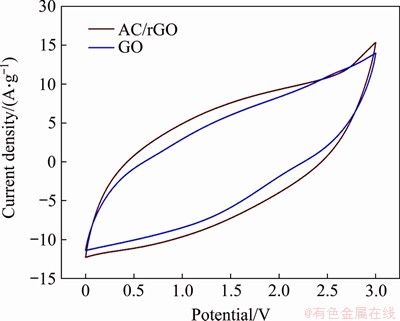
Figure 8 CV curves of rGO film and AC/rGO film at scan rate of 300 mV/s

Figure 9 XPS survey spectra of AC/rGO before (a) and after (b) 30000-cycle operations; High resolution XPS spectra of AC/rGO after 30000-cycle operations C 1s (c) and O 1s (d), before 30000-cycle operations C 1s (e) and O 1s (f)
4 Conclusions
AC particles have been successfully sandwiched between rGO sheets via a facile self-assemble approach, leading to the formation of free-standing and flexible AC/rGO film supercapacitor electrode. Since AC particles effectively suppressed the rGO sheet restacking, AC/rGO film exhibits loose layer-by-layer stacking structures, which is different from compact layer-by-layer stacking structures of pure graphene films. The as-formed gaps between AC particles and rGO sheets provide fast diffusion channels for electrolyte ions and enhanced accessible surface area of rGO, which is favorable for electrical double layers formation. Thanks to the sandwiched structure, the AC/rGO composite film delivers high specific capacitance and long cycle life in the voltage range of 0.0-3.0 V. This work offers a promising strategy to design free-standing supercapacitor electrodes using traditional nanocarbon materials.
Contributors
ZHU Lian-wen, GU Li and LU Shao-rong conceived and designed the study. WU Yuan-yuan performed the experiments. ZHU Lian-wen edited the draft of manuscript. CHEN Ru-ting, GU Li, CAO Xue-bo and LU Shao-rong reviewed the whole manuscript. All authors replied to reviewer & apos; s comments and revised the final version.
Conflict of interest
WU Yuan-yuan, ZHU Lian-wen, CHEN Ru-ting, GU Li, CAO Xue-bo and LU Shao-rong declare that they have no conflict of interest.
References
[1] WANG Xian-fu, LU Xi-hong, LIU Bin, CHEN Di, TONG Ye-xiang, SHEN Guo-zhen. Flexible energy-storage devices: Design consideration and recent progress [J]. Advanced Materials, 2014, 26(28): 4763-4782. DOI: 10.1002/adma. 201400910.
[2] LIU Wei, SONG Min-sang, KONG Biao, CUI Yi. Flexible and stretchable energy storage: Recent advances and future perspectives [J]. Advanced Materials, 2017, 29(1): 1603436- 1603469. DOI: 10.1002/adma.201603436.
[3] LI Lin, WU Zhong, YUAN Shuang, ZHANG Xin-bo. Advances and challenges for flexible energy storage and conversion devices and systems [J]. Energy Environmental Science, 2014, 7(7): 2101-2122. DOI: 10.1039/c4ee00318g.
[4] CONWAY B E. Electrochemical supercapacitors: Scientific fundamentals and technological applications [M]. Kluwer, The Netherlands: Springer, 1999.
[5] MILLER J R, SIMON P. Electrochemical capacitors for energy management [J]. Science, 2008, 321(5889): 651-652. DOI: 10.1126/science.1158736.
[6] SIMON P, GOGOTSI Y. Materials for electrochemical capacitors [J]. Nature Materials, 2008, 7(11): 845-854. DOI: 10.1038/nmat2297.
[7] LIU Li, FENG Yu, WU Wei. Recent progress in printed flexible solid-state supercapacitors for portable and wearable energy storage [J]. Journal of Power Sources, 2019, 410-411(15): 69-77. DOI: 10.1016/j.jpowsour.2018.11.012.
[8] WANG Guo-ping, ZHANG Lei, ZHANG Jun-jun. A review of electrode materials for electrochemical supercapacitors [J]. Chemical Society Reviews, 2012, 41(2): 797-828. DOI: 10.1039/c1cs15060j.
[9] GONZALEZ A, GOIKOLEA E, BARRENA J A, MYSYK R. Review on supercapacitors: technologies and materials [J]. Renewable and Sustainable Energy Reviews, 2016, 58: 1189-1206. DOI: 10.1016/j.rser.2015.12.249.
[10] HUANG Yi, LIANG Jia-jie, CHEN Yong-sheng. An overview of the applications of graphene-based materials in supercapacitors [J]. Small, 2012, 8(12): 1805-1834. DOI: 10.1002/smll.201102635.
[11] STOLLER M D, PARK S J, ZHU Yan-wu, AN J H, RUOFF R S. Graphene-based ultracapacitors [J]. Nano Letters, 2008, 8(10): 3498-3502. DOI: 10.1021/nl802558y.
[12] GWON H, KIM H S, LEE K U, SEO D H, PARK Y C, LEE Y S. Flexible energy storage devices based on graphene paper [J]. Energy and Environmental Science, 2011, 4(4): 1277-1280. DOI: 10.1039/C0EE00640H.
[13] EL-KADY M F, SHAO Yuan-long, KANER R B. Graphene for batteries, supercapacitors and beyond [J]. Nature Reviews Materials, 2016, 1(7): 16033. DOI: 10.1038/ natrevmats.2016.33.
[14] GUO Xiao-tian, ZHENG Sha-sha, ZHANG Guang-xun, XIAO Xiao, LI Xin-ran, XU Yu-xia, XUE Huai-guo, PANG Huan. Nanostructured graphene-based materials for flexible energy storage [J]. Energy Storage Materials, 2017, 9: 150-169. DOI: 10.1016/j.ensm.2017.07.006.
[15] YANG Zhou-fei, TIAN Jia-rui, YIN Ze-fang, CUI Chao-jie, QIAN Wei-zhong, WEI Fei. Carbon nanotube- and graphene-based nanomaterials and applications in high- voltage supercapacitor: A review [J]. Carbon, 2019, 141: 467-480. DOI: 10.1016/j.carbon.2018.10.010.
[16] CAO Xue-bo, QI Dian-peng, YIN Sheng-yan, BU Jing, LI Feng-ji, GOH C F, ZHANG Sam, CHEN Xiao-dong. Ambient fabrication of large-area graphene films via a synchronous reduction and assembly strategy [J]. Advanced Materials, 2013, 25(21): 2957-2962. DOI: 10.1002/adma. 201300586.
[17] LIU Gong-ping, JIN Wan-qin, XU Nan-ping. Graphene- based membranes [J]. Chemical Society Reviews, 2015, 44(15): 5016-5030. DOI: 10.1039/C4CS00423J.
[18] WU Qiong, XU Yu-xi, YAO Zhi-yi, LIU An-ran, SHI Gao-quan. Supercapacitors based on flexible graphene/ polyaniline nanofiber composite films [J]. ACS Nano, 2010, 4(4): 1963-1970. DOI: 10.1021/nn1000035.
[19] SHI Qiu-rong, CHA Y W, SONG Yang, LEE J I, ZHU Cheng-zhou, LI Xiao-yu, SONG M K, DU Dan, LIN Yue-he. 3D graphene-based hybrid materials: Synthesis and applications in energy storage and conversion [J]. Nanoscale, 2016, 8(34): 15414-15447. DOI: 10.1039/C6NR04770J.
[20] LU Xiang-jun, DOU Hui, GAO Bo, YUAN Chang-zhou, YANG Su-dong, HAO Liang, SHEN Lai-fa, ZHANG Xiao-gang. A flexible graphene/multiwalled carbon nanotube film as a high performance electrode material for supercapacitors [J]. Electrochimica Acta, 2011, 56(14): 5115-5121. DOI: 10.1016/j.electacta.2011.03.066.
[21] SUN Yi-qing, WU Qiong, XU Yu-xi, BAI Hua, LI Chun, SHI Gao-quan. Highly conductive and flexible mesoporous graphitic films prepared by graphitizing the composites of graphene oxide and nanodiamond [J]. Journal of Materials Chemistry, 2011, 21(20): 7154-7160. DOI: 10.1039/ c0jm04434b.
[22] SARKER A K, HONG J D. Layer-by-layer self-assembled multilayer films composed of graphene/polyaniline bilayers: High-energy electrode materials for supercapacitors [J]. Langmuir, 2012, 28(34): 12637-12646. DOI: 10.1021/la3021589.
[23] WANG Dong-niu, YANG Jin-li, LI Xi-fei, GENG Dong-sheng, LI Ru-ying, CAI Mei, SHAM T K, SUN Xue-liang. Layer by layer assembly of sandwiched graphene/SnO2 nanorod/carbon nanostructures with ultrahigh lithium ion storage properties [J]. Energy Environmental Science, 2013, 6(10): 2900-2906. DOI: 10.1039/ c3ee40829a.
[24] SONG Wei-li, CAO Mao-sheng, LU Ming-ming, BI Song, WANG Chan-yuan, LIU Jia, YUAN Jie, FAN Li-zhen. Flexible graphene/polymer composite films in sandwich structures for effective electromagnetic interference shielding [J]. Carbon, 2014, 66: 67-76. DOI: 10.1016/j.carbon.2013. 08.043.
[25] YU Ding-shan, DAI Li-ming. Self-assembled graphene/ carbon nanotube hybrid films for supercapacitors [J]. Journal of Physical Chemistry Letters, 2010, 1(2): 467-470. DOI: 10.1021/jz9003137.
[26] LI Xing, TANG Yao, SONG Jun-hua, YANG Wei, WANG Ming-shan, ZHU Cheng-zhou, ZHAO Wen-gao, ZHENG Jian-ming, LIN Yue-he. Self-supporting activated carbon/ carbon nanotube/reduced graphene oxide flexible electrode for high performance supercapacitor [J]. Carbon, 2018, 129: 236-244. DOI: 10.1016/j.carbon.2017.11.099.
[27] SONG Li, CAO Xue-bo, LI Lei, WANG Qiao-di, YE Hua-ting, GU Li, MAO Chang-jie, SONG Ji-ming, ZHANG Sheng-yi, NIU He-lin. General method for large-area films of carbon nanomaterials and application of a self-assembled carbon nanotube film as a high-performance electrode material for an all-solid-state supercapacitor [J]. Advanced Functional Materials, 2017, 27(21): 1700474. DOI: 10.1002/ adfm.201700474.
[28] YE Gui, YU Zhi-yong, LI Yi-ming, LI Lei, SONG Li, GU Li, CAO Xue-bo. Efficient treatment of brine wastewater through a flow-through technology integrating desalination and photocatalysis [J]. Water Research, 2019, 157: 134-144. DOI: 10.1016/j.watres.2019.03.058.
[29] LIU Piao, HE Wen-qiang, LU An-xian. Preparation of low-temperature sintered high conductivity inks based on nanosilver self-assembled on surface of graphene [J]. Journal of Central South University, 2019, 26: 2953-2960. DOI: 10.1007/s11771-019-4227-z.
[30] ZHOU Xiao-zhong, LU He-jie, TANG Xing-chang, ZENG Ya-ping, YU Xin. Facile synthesis of Sb@Sb2O3/reduced graphene oxide composite with superior lithium-storage performance [J]. Journal of Central South University, 2019, 26: 1493-1502. DOI: 10.1007/s11771-019-4105-8.
[31] OU Xing, YANG Cheng-hao, XIONG Xun-hui, ZHENG Feng-hua, PAN Qi-chang, JIN Chao, LIU Mei-lin, HUANG Kevin. A new rGO-overcoated Sb2Se3 nanorods anode for Na+ battery: In situ x-ray diffraction study on a live sodiation/desodiation process [J]. Advanced Functional Materials, 2017, 27(13): 1606242. DOI: 10.1002/adfm. 201606242.
[32] ZONG Meng, HUANG Ying, ZHAO Yang, SUN Xu, QU Chun-hao, LUO Di-di, ZHENG Jiang-bo. Facile preparation, high microwave absorption and microwave absorbing mechanism of RGO–Fe3O4 composites [J]. RSC Advances, 2013, 3(45): 23638-23646. DOI: 10.1039/C3RA43359E.
[33] DRESSELHAUS M S, JORIO A, HOFMAN M, DRESSELHAUS G, SAITO R. Perspectives on carbon nanotubes and graphene Raman spectroscopy [J]. Nano Letters, 2010, 10(3): 751-758. DOI: 10.1021/nl904286r.
[34] WU Shu-xing, LIU Can-bin, DINH D A, HUI K S, HUI K N, YUN J M, KIM K H, Three-dimensional self-standing and conductive MnCO3@graphene/CNT networks for flexible asymmetric supercapacitors [J]. ACS Sustainable Chemistry Engineering, 2019, 7(11): 9763-9770. DOI: 10.1021/acssuschemeng.8b05935.
[35] ZHANG Li-li, ZHAO Xin, STOLLER M D, ZHU Yan-wu, JI Heng-xing, MURALI S, WU Ya-ping, PERALES S, CLEVENGER B, RUOFF R S. Highly conductive and porous activated reduced graphene oxide films for high-power supercapacitors [J]. Nano Letters, 2012, 12(4): 1806-1812. DOI: 10.1021/nl203903z.
(Edited by YANG Hua)
中文导读
用于对称型超级电容器的自组装活性炭插层石墨烯膜
摘要:本文报道了活性炭插层石墨烯膜的自组装制备方法。制备的复合碳膜兼具自支撑、柔性和出色力学强度,可按需无损转移到各种基材上。由于活性炭颗粒有效抑制了石墨烯片的层层堆积,因此复合碳膜展现出疏松多孔的层状结构,这与石墨烯薄膜的致密结构不同。碳膜层间多孔结构为电解质离子提供快速扩散通道,并增加石墨烯膜的可利用表面积。因此,复合碳膜电极在0.0~3.0 V的电压范围内呈现出高比电容和出色的循环性能。本研究为使用传统碳材料制造高性能柔性电极,提供了一种简单高效的方法。
关键词:石墨烯膜;活性炭;自组装;超级电容器
Foundation item: Project(21673102) supported by the National Natural Science Foundation of China; Projects(LY18B010006, LQ19B030005) supported by the Natural Science Foundation of Zhejiang Province, China
Received date: 2020-04-11; Accepted date: 2020-09-04
Corresponding author: ZHU Lian-wen, PhD, Associate Professor; Tel: +86-13736492917; E-mail: lwzhu@zjxu.edu.cn; ORCID: https://orcid.org/0000-0002-6820-1381; GU Li, PhD, Professor; Tel: +86-13738299715; E-mail: guli@zjxu.edu.cn; ORCID: https://orcid.org/0000-0003-2003-3486; LU Shao-rong, PhD, Professor; Tel: +86-13788743571; E-mail: lushaor@163.com; ORCID: https://orcid.org/0000-0003-0376-1182

