文章编号:1004-0609(2016)-03-0681-08
硫化铜与铵盐焙烧反应行为
于云鹤1,田 原1,朱成席1,孙晨藤2,许 茜2
(1. 东北大学 材料与冶金学院,沈阳 110819;
2. 上海大学 省部共建高品质特殊钢冶金与制备国家重点实验室,上海 200072)
摘 要:通过热分析、X射线衍射分析和红外光谱分析等手段对CuS与NH4Cl或(NH4)2SO4的焙烧过程进行研究,并对焙烧产物中铜元素的水浸出率进行测定。结果表明:CuS与NH4Cl焙烧过程中,在NH4Cl分解之前,CuS会与NH4Cl反应生成铜铵化合物;在空气中焙烧CuS+NH4Cl,CuS会分解成Cu2S;焙烧最终产物为微溶于水的CuCl,使焙烧产物铜水浸率较低。CuS+(NH4)2SO4在空气气氛下焙烧,在焙烧温度不低于300 ℃焙烧1 h以上时,产物主要是铜的硫酸盐,其中铜元素可以完全浸出;空气中的氧参与焙烧反应,促进(NH4)2SO4的分解和CuS的转化;焙烧过程中出现亚硫酸铜铵和硫酸铜铵的中间产物。同时,还对比分别用NH4Cl和(NH4)2SO4焙烧CuS的差异。
关键词:硫化铜;氯化铵;硫酸铵;反应机理;焙烧反应;水浸出
中图分类号:TF111 文献标志码:A
金属铜在电子、电力传输、机械、军工、建筑等有着重要和广泛的应用[1]。铜的生产方式有两种,一种是火法炼铜,另一种是湿法炼铜。目前,火法冶金是铜的主要生产方式,铜含量为0.5%~2%(质量分数)时的铜矿石经过选矿之后,可以富集至8%~35%(质量分数)硫化铜精矿;经过熔炼、吹炼、精炼等工序,得到粗铜;粗铜经过电解精炼之后得到含铜99.95%(质量分数)金属铜。随着高品位铜矿资源的日益减少,低品位硫化矿、复杂多金属伴生铜矿、低品位氧化铜矿及氧化物/硫化物混合矿将成为今后炼铜的主要资源。这类贫矿是很难直接通过火法冶金过程处理的,使铜的湿法冶金过程的重要性逐渐凸显[2]。铜湿法冶金过程中,将铜元素从矿相中浸出、使其进入溶液相是重要的环节之一。无论是采用酸浸出、还是氨法浸出[3-4],铜的氧化物是较容易进入溶液相的,而铜的硫化物通常是较难直接浸出的;一般需要经矿相转化、其他离子替换硫化物中的硫离子之后,才能使其中的铜元素具有可溶性。对于碱性脉石含量较高的铜镍矿,最近冶金学家提出采用铵盐焙烧[5-7],选择性地将铜或镍的氧化物和硫化物转化为可溶性的氯化物,可以选择地将铜[8-9]、镍[10-12]和钴[13-14]等从矿石中分离出来,避免了大量的碱性脉石进入浸出液[15-17],使后续的湿法冶金过程变得简单易行。但是,上述冶金方法对于含较多硫化物的矿相将面临挑战,其中的硫化铜能否有效地转化为可溶性的盐,将是该冶金工艺能否高效利用氧化/硫化物混合矿物中铜元素的关键。
本文作者以硫化铜为对象,研究了其在氯化铵和硫酸铵焙烧过程中的反应机理、焙烧最终产物的组成以及水浸行为,为采用铵盐焙烧处理低品位硫化矿、以及氧化/硫化物共生的铜矿提供理论依据,并且寻求强化铵盐焙烧过程的有效途径。
1 实验
实验使用分析纯试剂CuS、NH4Cl以及(NH4)2SO4,购自国药集团化学试剂有限公司。
硫化铜的焙烧实验在带有不锈钢反应器的SG-1.5-9型坩埚电阻炉内进行。不锈钢反应器带有排气口,焙烧尾气经过H2SO4溶液和NaOH水溶液吸收后排空。按一定质量配比分别称取硫化铜、氯化铵或硫酸铵,于研钵内研磨均匀后放入瓷坩锅中;将瓷坩埚放入不锈钢反应器,置于电阻炉中;利用KSY-6D-16型单相可控硅温度控制器使物料在选定温度下焙烧至设定时间。对焙烧熟料进行水浸出实验,将过滤所得浸出液配制成250 mL溶液,并通过碘量法(GBT 3884.1-2012)测定溶液中铜离子含量,从而计算铜的浸出率。实验流程如图1所示。
按以下公式计算浸出率:
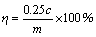 (1)
(1)
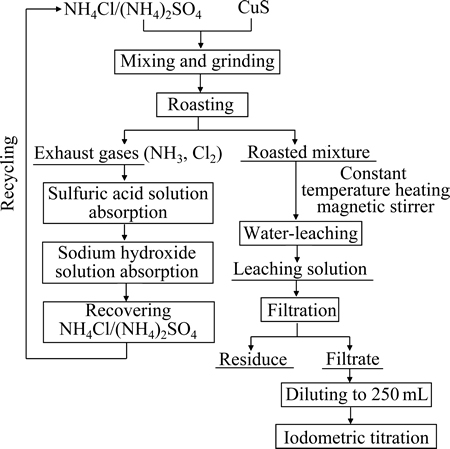
图1 实验流程图
Fig. 1 Flow diagram of experiment
式中:c为浸出液中铜离子的浓度,g/L;m为人造矿中金属的质量,g;η为浸出率,%。
取一定量的焙烧熟料,利用日本理学D/max- 2500PC型X射线衍射仪(Cu 辐射)进行物相分析。用Nicolet 380 FT-IR型智能傅立叶红外光谱仪对一定量的焙烧熟料进行红外光谱分析。此外,对硫化铜与铵盐的焙烧过程进行热分析,取CuS和NH4Cl、或(NH4)2SO4混合物(约10 mg) 放入刚玉坩埚中,使用SDT Q600型热分析仪,以10 ℃/min升温速率由室温加热到设定温度,气流量为100 mL/min。
2 结果与讨论
2.1 CuS与NH4Cl的焙烧行为
将CuS分别在空气和氮气气氛下与NH4Cl的混合物一起焙烧,研究了从室温至500 ℃过程中的质量损失和热流量的变化。为了便于比较,将氯化铵热分解热重分析结果也一并放入图2中。
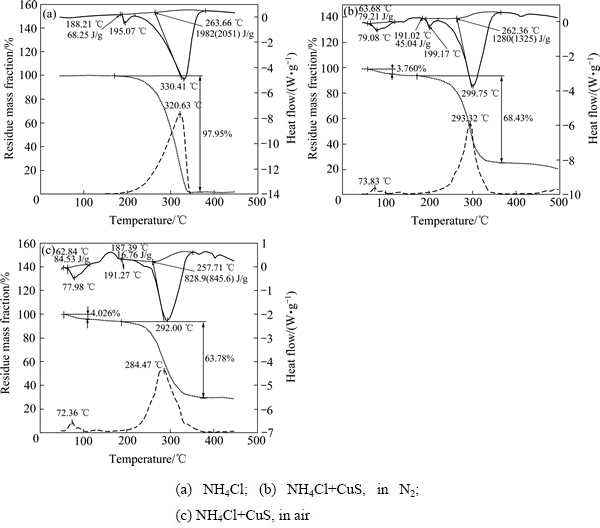
图2 NH4Cl和CuS+NH4Cl不同条件下的热重-差热分析曲线
Fig. 2 TG-DSC curves of NH4Cl and CuS+NH4Cl under different conditions
实验结果表明,NH4Cl在188 ℃左右开始一个晶型的转变[18],对应了一个吸热峰;约200 ℃开始质量损失过程,在315 ℃左右,质量损失速率最快;340 ℃时,NH4Cl基本完成分解过程。
NH4Cl+CuS在氮气中焙烧的热重曲线显示,其质量损失速率增减与温度的对称性明显好于NH4Cl的热分解,且在约293 ℃质量损失速率最快。结合BORISOV等[19]的研究结果,氮气气氛中NH4Cl在完全分解之前会与CuS发生反应;最终NH4Cl会释放出所有的NH3。反应方程见式(3)和(4),由式(3)和(4)可以看出,在NH4Cl分解反应的初期,CuCl2结合了部分NH4Cl;而在反应的后期又将结合的NH4Cl释放,并且NH4Cl会完成分解,使其质量损失速率增减在温度293 ℃附近呈现对称性。
图2(c)所示为空气气氛中NH4Cl+CuS的焙烧反应,结果显示空气气氛中该反应的孕育过程明显短于氮气气氛中的相关反应,这反映出氧的介入有助于氯离子替换CuS中的硫离子。结合不同温度下CuS与NH4Cl在空气中焙烧产物的XRD分析结果(见图3),推断空气气氛中CuS会分解为Cu2S,同时Cu(Ⅱ)也可能被低价的硫离子还原成Cu(Ⅰ)。空气中CuS+ NH4Cl焙烧反应造成的质量损失率略低于氮气气氛中CuS+NH4Cl的质量损失率,很可能是由于最终产物中包含少量亚硫酸铜或硫酸铜。
根据文献[19]和不同焙烧温度下的焙烧产物XRD分析(见图3),焙烧过程中CuS空气中的分解反应见等式(2),在整个焙烧温度范围内,ΔG<0。
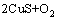 →
→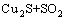 (2)
(2)
在进一步的焙烧中,Cu离子可以与Cl离子形成配合离子,得到焙烧的中间产物NH4CuCl3(见式(3))。氯化铜铵是不稳定的复合物,超过200 ℃以上的焙烧温度会使其分解为CuCl2(见式(4))。图3中CuCl由Cu2S与未反应的NH4Cl反应生成的,反应如等式(5)所示,该反应在焙烧温度内ΔG<0,可以自发进行。
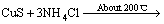 NH4CuCl3+H2S+2NH3 (3)
NH4CuCl3+H2S+2NH3 (3)
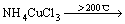
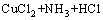 (4)
(4)
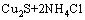 =
=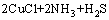 (5)
(5)
本文作者也研究不同温度下焙烧2 h产物的水浸出特征(见图4),发现焙烧温度为280 ℃时,水浸出率约为26%。随着焙烧温度升高,焙烧产物的水浸率下降。这是由于随着焙烧温度的升高,产物中CuCl含量增加,而CuCl仅仅微溶于水的。同时,CuCl露置在空气中会部分氧化成碱式盐[20],这也是造成铜浸出率低的原因之一。
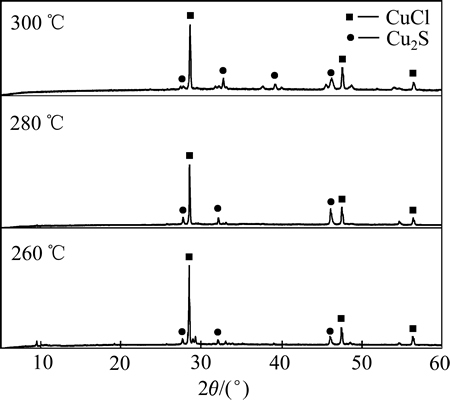
图3 不同温度下CuS+NH4Cl在空气中焙烧产物的XRD谱
Fig. 3 XRD patterns of product of CuS+NH4Cl roasted at different temperatures in air
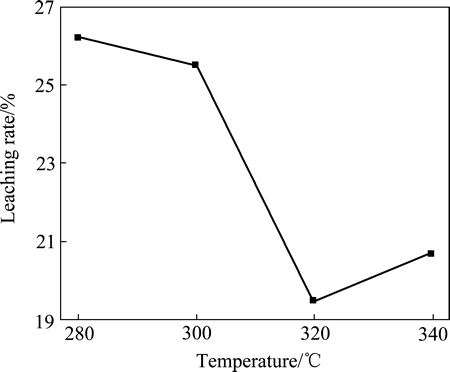
图4 焙烧温度对焙烧产物铜浸出率的影响
Fig. 4 Effect of roasting temperature on leaching rate of copper
2.2 CuS与(NH4)2SO4的焙烧行为
根据本实验结果和文献[21-22]可以分析(NH4)2SO4热分解过程的3个阶段。在200~320 ℃之间,(NH4)2SO4基本完成等式(6)的反应,
(NH4)2SO4→NH4HSO4+NH3 (6)
当温度进一步升高至330 ℃,发生进一步的分解(见式(7))。
2 NH4HSO4→(NH4)2S2O7+H2O (7)
温度升高到近400 ℃以后,
3(NH4)2S2O7→2NH3+2NH2+6SO2+9H2O (8)
图5(a)所示为(NH4)2SO4在空气气氛下焙烧的热重分析结果,在整个焙烧过程中有两个明显的吸收峰,随着温度的升高质量损失不断增加,在301.93 ℃和411.47 ℃时,质量损失速率分别达到两个峰值,本实验得到的结果与上述文献中的结果一致。
图5(b)所示为(NH4)2SO4+CuS在空气气氛下焙烧的热重分析结果。在200~320 ℃第一阶段分解中,(NH4)2SO4+CuS混合物的质量损失率高于(NH4)2SO4热分解的质量损失率,且微商热重曲线在260 ℃出现一个小峰,这表明在这一阶段中,除了(NH4)2SO4分解外,还会有其它的质量损失反应发生,如CuS的氧化还原反应见式(2),并可能进一步转化为(NH4)2Cu(SO4)2。
当焙烧温度进一步升高至300 ℃时,(NH4)2SO4+CuS混合物的质量损失速率远高于(NH4)2SO4的;结合(NH4)2SO4+CuS在300 ℃焙烧2 h产物的XRD结果可知(见图6),经过焙烧后,CuS主要转化为硫酸盐,是一个增加过程,这说明由于CuS的加入加速了硫酸铵的分解过程。
图5(c)所示为(NH4)2SO4+CuO在空气气氛下焙烧的热重分析结果。由于CuO在整个焙烧过程中都不会产生质量损失,而且(NH4)2SO4+CuO焙烧过程中质量的变化与(NH4)2SO4热分解表现的行为非常类似, 这表明CuO的加入对(NH4)2SO4热分解反应的影响不大。但是(NH4)2SO4热分解过程中释放的NH3可能被生成的CuSO4化学吸附,生成Cu(NH3)xSO4,会减缓最初的质量损失速率。
图6所示为不同焙烧温度下(NH4)2SO4+CuS的焙烧产物。随着焙烧温度逐渐升高至300 ℃,CuS依次转变为(NH4)2Cu(SO4)2和(NH4)2Cu3(SO4)4。硫酸铜铵也是不稳定的配合物,提高焙烧温度可以将其分解成硫酸铜,见式(9)和(10)。
2CuS+4(NH4)2SO4+3O2→2(NH4)2Cu(SO4)2+4NH3+2SO2+2H2O (9)
(NH 4)2Cu(SO4)2→CuSO4+NH3+SO2+H2O+0.5O2 (10)
本文作者还研究(NH4)2SO4+CuS在280 ℃焙烧不同时间的产物(见图7)。当焙烧0.5 h时,反应进行程度较小,大部分的Cu仍以不溶的硫化物形式存在;当焙烧时间到达1 h以上时,大部分的铜都反应转化为可溶性的硫酸盐。明显看出(NH4)2SO4分解不完全,出现分解中间产物之一的NH4HSO4;CuS在最终转变为(NH4)2Cu3(SO4)4之前,存在(NH4)2Cu(SO4)2等中间产物。
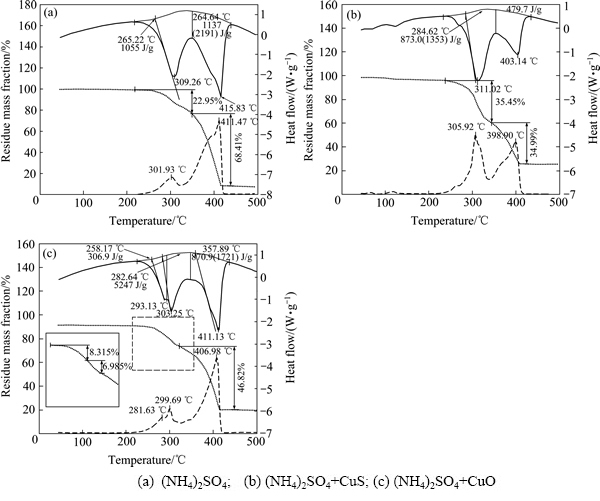
图5 (NH4)2SO4、(NH4)2SO4+CuS和(NH4)2SO4+CuO在空气气氛中的热重和差热分析曲线
Fig. 5 TG-DSC curves in air

图6 不同温度下CuS+(NH4)2SO4在空气中焙烧2 h后产物的XRD谱
Fig. 6 XRD patterns of product of CuS+(NH4)2SO4 roasted at different temperatures in air for 2 h
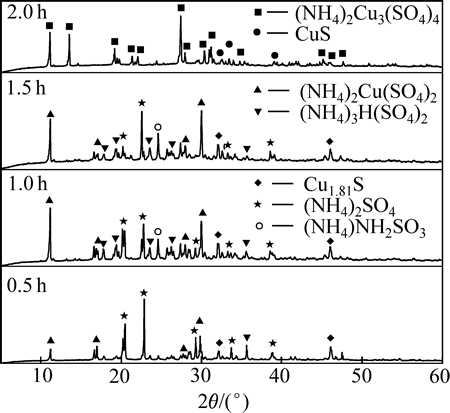
图7 空气中CuS+(NH4)2SO4焙烧不同时间产物的XRD谱
Fig. 7 XRD patterns of product of CuS+(NH4)2SO4 roasted for different times in air
为了进一步解释焙烧反应的内在机理,分别取未焙烧的(NH4)2SO4+CuS和300 ℃下焙烧2 h的焙烧产物作红外光谱图,其结果如图8所示。
由于CuS属于低红外光活性化合物,在(NH4)2SO4+CuS混合物的红外谱主要呈现了(NH4)2SO4的特征[23]。1400 cm-1以上的吸收峰体现的是(NH4+)振动行为,约3220 cm-1附近是非对称伸缩振动,3044 cm-1对称性伸缩振动;约1700 cm-1对称性变形振动,约1417 cm-1是非对称性变形振动。在1400 cm-1以下是 (SO42-)振动模式,对应1093 cm-1附近是非对称伸缩,616 cm-1附近非平面变形振动。对于(NH4)2SO4+CuS焙烧之后的产物,所有 (NH4+)振动模式均向高波数方向移动。由于焙烧产物XRD分析表明其中含有硫酸铜铵,红外光谱的结果表明:由于Cu离子的引入使得(NH4+)内部的化学键键能增强。焙烧产物的红外光谱显示原有的硫酸铵中(SO42-)振动模式基本保持在原来位置,但是会出现吸收峰的劈裂现象,这可能是由于硫酸铜铵中同时含有Cu(Ⅱ,Ⅰ)以及(NH4+)功能团改变了原有硫酸铵中(SO42-)的能级分布状态,造成能级的裂分。还有一种观点认为金属某些金属离子可以硫酸根离子形成多齿配位结合[24],即铜离子与硫酸根离子的结合更牢固;如果焙烧温度升高,会释放铵根离子而生成硫酸铜,也证实这一观点的合理性。
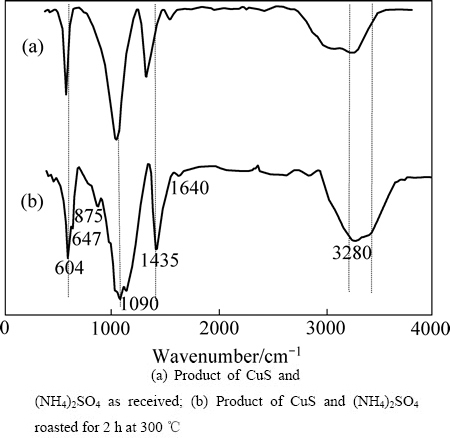
图8 不同样品的红外光谱
Fig. 8 Infrared spectra of samples
图9所示为不同温度下焙烧产物铜浸出率。由图9可看出,3个焙烧温度下铜的浸出率随焙烧时间变化趋势基本相同。随着焙烧时间的增加,铜浸出率不断提高,直至完全浸出(100%)。与NH4Cl焙烧产物相比,利用(NH4)2SO4和CuS焙烧可以将CuS完全转化为硫酸盐,实现铜的完全浸出;且焙烧温度越高,完全转化成硫酸盐所需时间越短;当焙烧温度不低于 300 ℃时,焙烧1.0 h即可几乎将铜全部浸出。
与氯化铵焙烧相比较,在控制温度的焙烧条件下,CuS+(NH4)2SO4焙烧最终产物是硫酸铜,其在空气条件下可以稳定存在,且易溶于水。如果基于良好浸出,(NH4)2SO4是将CuS转化为硫酸铜的良好选择。CuS与氯化铵的焙烧产物最终产物是CuCl,尽管该产物微溶于水;但是,其可以溶解于NaCl或KCl溶液形成配合离子CuCl2-,进一步电沉积铜时与Cu2+相比,可以节省一半的电量。因此,有必要进行进一步的深入研究。
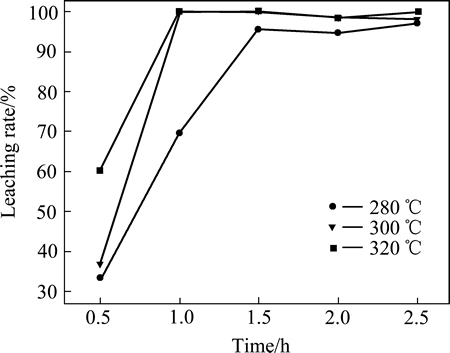
图9 不同温度下焙烧产物的铜浸出率
Fig. 9 Copper leaching rate of roasted products at different temperatures for different time
3 结论
1) CuS+NH4Cl无论是在空气和氮气气氛下焙烧,最终的焙烧产物主体产物为CuCl;在NH4Cl分解之前,CuS会与NH4Cl反应生成铜铵化合物;空气中焙烧CuS+NH4Cl,CuS还会与氧反应,生成Cu2S。CuS+NH4Cl焙烧产物的铜浸出率较低是由于焙烧产物CuCl微溶于水和其挥发性。
2) CuS+(NH4)2SO4在空气气氛下焙烧,当焙烧温度不低于300 ℃、焙烧1 h以上时,产物主要是铜的硫酸盐;空气中的氧会参与焙烧反应,促进(NH4)2SO4的分解和CuS的转化;焙烧过程中出现硫酸氢铵、硫酸铜铵等中间产物;硫酸铜铵中由于铜离子的引入增强铵根离子中的结合键能。同时,也可能裂化硫酸根离子内的能级;经过焙烧的CuS+(NH4)2SO4可以实现铜元素的完全水浸出。
REFERENCES
[1] 周 平, 唐金荣, 施俊法, 杨宗喜, 金 玺. 铜资源现状与发展态势分析[J]. 岩石矿物学杂志, 2012, 31(5): 750-756.
ZHOU Ping, TANG Jin-rong, SHI Jun-fa, YANG Zong-xi, JIN Xi. Analysis of status and development trend of copper resources[J]. Acta Petrologica et Mineralogica, 2012, 31(5): 750-756.
[2] 刘志雄, 尹周澜, 胡慧萍, 陈启元. 低品位氧化铜矿氨-硫酸铵体系过硫酸铵氧化浸出[J]. 中国有色金属学报, 2012, 22(5): 1488-1496.
LIU Zhi-xiong, YIN Zhou-lan, HU Hui-ping, CHEN Qi-yuan. Oxidative leaching of low-grade copper ore in ammonia-ammonium sulfate solution with ammonium persulfate[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(5): 1488-1496.
[3] 程 琼, 章晓林, 刘殿文, 张文彬. 某高碱性氧化铜矿常温常压氨浸试验研究[J]. 湿法冶金, 2006, 25(2): 74-77.
CHENG Qiong, ZHANG Xiao-lin, LIU Dian-wen, ZHANG Wen-bin. Ammonia leaching of oxidized copper ore at normal temperature and pressure[J]. Hydrometallurgy, 2006, 25(2): 74-77.
[4] 杨新生. 氨浸过程浅析[J]. 有色矿冶, 1993(1): 34-38.
YANG Xin-sheng. Analysis of ammonia leaching process[J]. Nonferrous Mining and Metallurgy, 1993(1): 34-38.
[5] ZHANG Ming-zhu, ZHU Guo-cai, ZHAO Yu-na, FENG Xiu-juan. A study of recovery of copper and cobalt from copper-cobalt oxide ores by ammonium salt roasting[J]. Hydrometallurgy, 2012, 129/130: 140-144.
[6] CHEN Ci-yun, WEN Shu-ming, ZHAO He-fei,  Chao, WANG Yi-jie. Using of roasting leaching methods for copper recovery from a flotation concentrate of Yangla copper ore[J]. Advanced Materials Research, 2013, 868: 403-407.
Chao, WANG Yi-jie. Using of roasting leaching methods for copper recovery from a flotation concentrate of Yangla copper ore[J]. Advanced Materials Research, 2013, 868: 403-407.
[7] LIU Xin-wei, FENG Ya-li, LI Hao-ran, YANG Zhi-chao, CAI Zhen-lei. Recovery of valuable metals from a low-grade nickel ore using an ammonium sulfate roasting-leaching process[J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(5): 377-383.
[8] RASHID K N, LEILA I S, AISULU K Z, MURATBEK T U. Recovery of value metals from copper smelter slag by ammonium chloride treatment[J]. International Journal of Mineral Processing, 2013, 124: 145-149.
[9] 刘欣伟, 冯雅丽, 李浩然, 蔡震雷, 杨志超. 硫酸铵焙烧法浸出镍磁黄铁矿中有价金属[J]. 中国有色金属学报, 2012, 22(5): 1520-1526.
LIU Xin-wei, FENG Ya-li, LI Hao-ran, CAI Zhen-lei, YANG Zhi-chao. Leaching of valuable metals from nickel pyrrhotite by ammonium sulfate roasting method[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(5): 1520-1526.
[10] WANG Ming-yu, WANG Xue-wen. Extraction of molybdenum and nickel from carbonaceous shale by oxidation roasting, sulphation roasting and water leaching[J]. Hydrometallurgy, 2010, 102: 50-54.
[11] GUO Xue-yi, LI Dong, PARK K H, TIAN Qing-hua, WU Zhan. Leaching behavior of metals from a limonitic nickel laterite using a sulfation-roasting-leaching process[J]. Hydrometallurgy, 2009, 99: 144-150.
[12] 刘欣伟, 胡文韬, 李浩然, 冯雅丽. 高镁型低品位硫化镍矿浸出工艺及机理[J]. 中国有色金属学报, 2014, 24(6): 1599-1606.
LIU Xin-wei, HU Wen-tao, LI Hao-ran, FENG Ya-li. Leaching process and mechanism of high magnesium low-grade nickel sulfide ore[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(6): 1599-1606.
[13] PARK K H, MOHAPATRA D, REDDY B R, NAM C W. A study on the oxidative ammonia/ammonium sulphate leaching of a complex (Cu-Ni-Co-Fe) matte[J]. Hydrometallurgy, 2007, 86: 164-171.
[14] 石剑锋, 王志兴, 胡启阳, 郭华军, 李新海, 彭文杰. 硫酸氢铵硫酸化焙烧法红土镍矿提取镍钴[J]. 中国有色金属学报, 2013, 23(2): 510-515.
SHI Jian-feng, WANG Zhi-xing, HU Qi-yang, GUO Hua-jun, LI Xin-hai, PENG Wen-jie. Recovery of nickel and cobalt from nickel laterite ore by sulfation roasting method using ammonium bisulfate[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 510-515.
[15] 辛海霞, 吴 艳, 刘少名, 翟玉春. 硫酸铵-高铁铝土矿焙烧法提取铝、铁[J]. 矿产保护与利用, 2013(4): 37-40.
XIN Hai-xia, WU Yan, LIU Shao-ming, ZHAI Yu-chun. Recovery of aluminum and iron from high-iron bauxite by roasting method using ammoniumsulfate[J]. Conservation and Utilization of Mineral Resources, 2013(4): 37-40.
[16] 杨仲平, 靳晓珠, 朱国才. 铵盐焙烧法处理低品位锰矿的工艺研究[J]. 中国锰业, 2006, 24(3): 12-14.
YANG Zhong-ping, JIN Xiao-zhu, ZHU Guo-cai. Process of techniques researches on low-grade manganese ore by roasting with ammonium salt[J]. China’s Manganese Industry, 2006, 24(3): 12-14.
[17] 朱国才, 李赋屏, 肖明贵. 采用硫酸铵焙烧方法从低品位碳酸锰矿中富集回收锰[J]. 桂林工学院学报, 2005, 25(4): 534-537.
ZHU Guo-cai, LI Fu-ping, XIAO Ming-gui. Process of enriching and recovering Mn by roasting the low-grade manganese carbonate ore with ammonium sulfate[J]. Journal of Guilin University of Technology, 2005, 25(4): 534-537.
[18] 梅德清, 赵 翔, 王书龙, 袁银男, 孙 平. 柴油机排放颗粒物的热重特性分析[J]. 农业工程学报, 2013, 29(16): 50-56.
MEI De-qing, ZHAO Xiang, WANG Shu-long, YUAN Yin-nan, SUN Ping. Thermogravimetric characteristics analysis of particulate matter of emission of divided diesel[J]. Transactions of the Chinese Society of Agricultural Engineering, 2013, 29(16): 50-56.
[19] BORISOV V, D'YACHENKO A, KRAIDENKO R. Reaction of ammonium chloride with the copper(Ⅱ) sulfide and oxide, and identification of the reaction products[J]. Russian Journal of General Chemistry, 2011, 81: 1430-1433.
[20] 赵 伟. 氯化亚铜合成及精制[J]. 氯碱工业, 1999(4): 32-34.
ZHAO Wei. Synthesis and purification of cuprous chloride[J].Chlor-Alkali Industry, 1999(4): 32-34.
[21] 刘科伟, 陈天朗. 硫酸铵的热分解[J]. 化学研究与应用, 2002, 14(6): 737-739.
LIU Ke-wei, CHEN Tian-lang. Studies on the thermal decomposition of ammonium sulfate[J]. Chemical Research and Application, 2002, 14(6): 737-739.
[22] 范芸珠, 曹发海. 硫酸铵热分解反应动力学研究[J]. 高校化学工程学报, 2011, 25(2): 341-346.
FAN Yun-zhu, CAO Fa-hai. Thermal decomposition kinetics of ammonium sulfate[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(2): 341-346.
[23] BOURAHLA S, BENAMARA A A, MOUSTEFAI S K. Infrared spectra of inorganic aerosols: Ab initio study of (NH4)2SO4, NH4NO3, and NaNO3[J]. Canadian Journal of Physics, 2014, 92(3): 216-221.
[24] 魏长平, 柳 翱, 罗 靖. 固体硫酸铵-二氧化锆体系的红外光谱研究[J]. 纺织高校基础科学学报, 1996, 9(1): 43-46, 54.
WEI Chang-ping, LIU Ao, LUO Jing. IR investigation of solid mixture of (NH4)2SO4 and ZrO2[J]. Basic Sciences Journal of Textile Universities, 1996, 9(1): 43-46, 54.
Reaction behaviour of CuS during ammonium roasting
YU Yun-he1, TIAN Yuan1, ZHU Cheng-xi1, SUN Chen-teng2,XU Qian2
(1. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China;
2. State Key Laboratory of Advanced Special Steel, Shanghai University, Shanghai 200072, China)
Abstract: The reaction mechanism of ammonium chloride or sulfate roasting of CuS was investigated by employing thermogravimetric (TG), X-ray diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) analyses. The water-leaching procedure was carried out for the products from the roasting process, and the copper yield in the aqueous solution was determined by the method of iodometric titration. The results show that ammonium chloride can react with CuS before its thermal decomposition, and ammonium tetrachlorocuprate is found as an intermedia product. The final product of CuCl leads to the low leaching yield of copper. The ammonium sulfate roasting of CuS is carried on in the air at the temperature up to 300 ℃, leaves copper sulfate as the final product, and copper ammonium sulfate as the intermediates. All the copper in the roasted products can be dissolved into the aqueous solution in the post water-leaching. The difference of ammonium roasting of CuS was contrasted.
Key words: copper monosulfide; ammonium chloride; ammonium sulfate; reaction mechanism; roasting; water-leaching
Foundation item: Project(2014CB643405) supported by the National Basic Research Development Program of China
Received date: 2015-05-06; Accepted date: 2015-10-08
Corresponding author: XU Qian; Tel: +86-21-56338244; E-mail: qianxu@shu.edu.cn
(编辑 李艳红)
基金项目:国家重点基础研究发展计划资助项目(2014CB643405)
收稿日期:2015-05-06;修订日期:2015-10-08
通信作者:许 茜,教授,博士;电话021-56338244;E-mail:qianxu@shu.edu.cn