文章编号: 1004-0609(2005)03-0391-06
贮氢材料VHx(x=0, 1, 2)电子结构的DV-Xα研究
李 荣1, 2, 周上祺1, 梁国明2, 刘守平1, 陈昌国1
(1. 重庆大学 材料科学与工程学院, 重庆 400044;
2. 重庆师范大学 化学学院, 重庆 400047)
摘 要: 利用电荷自洽离散变分Xα(SCC-DV-Xα)方法计算了钒基固溶体中钒氢反应前后钒及其氢化物(VHx, x=0, 1, 2)的电子结构。 对净电荷、 键级和电子密度差的分析表明: VH和VH2中V—H键之间既有离子性又有共价性的相互作用。 VH2中V—H之间的键级比VH中V—H之间的键级小, 说明VH2中的H容易释放出来。 对能级结构、 态密度和价轨道集居数的分析表明, 氢化物VH中是V的4s轨道和H的1s轨道作用成键; 氢化物VH2中是V的4s、 3d轨道和H的1s轨道作用成键; VH比VH2的费米能级低, 说明VH更稳定。 解释了VH2中的氢不能全部放出的原因。
关键词: 钒氢化物; 电子结构; SCC-DV-Xα方法
中图分类号: O641.121 文献标识码: A
Quantum chemical DV-Xα study on electronic structure of hydrogen storage materials VHx(x=0, 1, 2)
LI Rong1, 2, ZHOU Shang-qi1, LIANG Guo-ming2, LIU Shou-ping1, CHEN Chang-guo1
(1. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. College of Chemistry, Chongqing Normal University, Chongqing 400047, China)
Abstract: The electronic structure of metallic V before and after reaction of V—H and its hydrides(VHx, x=0, 1, 2) in vanadium base solid solution was calculated by a quantum chemical method(SCC-DV-Xα). By analyzing net charge, bond level and electron density difference, the following results are obtained: there exists interaction between V—H bands interaction which has not only ionic but also covalent property in hydride VH and VH2, bond level between V—H in hydrideVH2 is weaker than that in VH, which proves that H in VH2 is released more easily than in VH. By analyzing the energy level structure and electron density of states and the electron occupation numbers in valence orbits, the result show that the former V 4s-H 1s interaction is bonding-type, and also the latter V 3d, 4s-H 1s interaction is bonding-type. Fermi energy level in VH is weaker than that in VH2, which proves that VH is more stabilization, and explains the reason why H2 is not all released.
Key words: vanadium hydride; electronic structure; SCC-DV-Xα method
由于传统的AB5、 AB2和AB型贮氢材料的贮氢量均低于2%(质量分数), 限制了贮氢材料在燃料电池上的应用, 故对于高容量的贮氢材料的研究倍受关注。 钒基固溶体材料作为一种新型的贮氢材料, 具有可逆贮氢量大、 氢在氢化物中的扩散速度较快等优点, 具有广阔的发展前景。 目前对钒基固溶体型贮氢材料的研究开发主要是镍氢电池用的V3TiNi0.56Mx(x=0.046~0.24, M为Al、 Si、 Mn、 Fe、 Co、 Zr……)等。 通过优化合金的相结构与组成进一步提高V基固溶体型材料的综合电化学性能, 以及研究开发价格低廉的V或V合金原材料以降低固溶体材料成本, 是目前V基固溶体型贮氢材料的两个主要研究方向。 钒基固溶体吸氢时可生成VH和VH2两种不同类型的氢化物, 在P-C-T曲线上出现两个相应的压力平台。 V基固溶体合金具有贮氢量大(约3.8%)的特点, 其气态吸放氢特性早在20世纪70年代已进行系统研究。 研究表明, 尽管由于V基固溶体氢化生成的VH型氢化物过于稳定而无法利用, 但就高平台对应的VH2→VH+H吸放氢特性来看, 合金的可逆贮氢量可达1.9%左右, 仍大于AB5、 AB2型贮氢材料。 钒基固溶体的吸氢量与固溶体中V的含量相关, 钒基固溶体贮氢合金与氢的反应主要是V与H2的反应, 因此对钒氢反应的研究非常重要。 有一些学者对此进行了实验研究[1-5]。
从理论上研究钒氢反应机理的学者如彭述明等[6]用局域密度泛函近似方法对金属钒及不同原子比的钒氢化物进行了结构优化和总能量计算; Matumura等[7]用DV-Xα方法研究了含各种合金元素的VH2和V2H的电子结构; Yukawa等[8, 9]用DV-Xα方法研究了LaNi5、 TiFe、 ZrMn2、 Mg2Ni和V的氢化物的电子结构, 表明控制其氢化物的稳定性的原子间相互作用在很大程度上取决于在氢化过程中晶体结构演变的方式。 但V吸氢生成VH, 以及VH吸氢生成VH2过程中, 氢化物的电子结构与贮氢性能的关系还未见报道。 本文作者应用量子化学DV-Xα方法研究V和H反应生成VH、 VH2过程中原子的净电荷、 能级结构、 态密度、 键级、 电子密度的变化, 探讨了它们与材料的贮氢性能之间的关系。
1 模型与方法
1.1 结构模型
本文应用“种子原子法”(seed atoms)[10], 选择 V原子作为中心原子, 不断地将其近邻原子按照远近顺序逐步包含到团簇中。 钒的晶体结构为体心立方晶格(bcc), 选取27个晶胞共91个原子作为晶族模型, 晶胞参数a=0.3029nm[7], 空间群为Td。 金属钒吸氢后, 其晶体结构发生了转变。 在两相区域(即a+β区域)内含氢量很低时形成体心四方晶格, 可以认为是略有扭转的体心四方晶格(bct), 考虑程序的计算能力, 选取59个V原子和56个H原子作为晶族模型, 其晶胞参数a=0.3001nm, c=0.3395nm[7], 空间群为D2h; VH2的晶体结构为面心立方晶格(fcc), 每个H原子占据晶体中的8c位置, 它是正四面体的中心。 选取63个V原子和64个H原子作为晶族模型, 晶胞参数a=0.4271nm[7], 空间群为Td。 VHx(x=0, 1, 2)的原子簇模型如图1所示, 模型中的数字表示空间群中原子的种类(结果与讨论中的数字与此相同)。
1.2 计算方法
DV-Xα(离散变分Xα)方法基于非相对论单电子Hamiltonian算符:
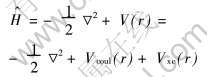
式中 库仑势Vcoul(r)是分子中各原子核对电子的吸引和电子之间的排斥势, 且
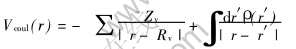
Vxc(r)是电子间的近似交换势:
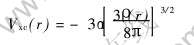
式中 α是交换常数, 取值范围通常为2/3≤α≤1, 本文计算中取α=0.7。 用LCAO-MO方法, 将分子波函数向原子轨道展开为 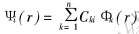 , 系数Cki可通过数值求解久期方程得到。
, 系数Cki可通过数值求解久期方程得到。
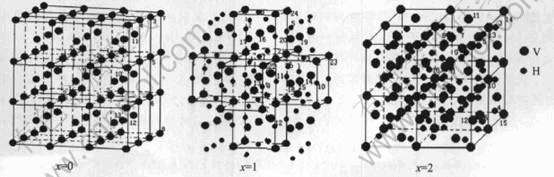
图1 VHx(x=0, 1, 2)的原子簇模型
Fig.1 Cluster modes of VHx(x=0, 1, 2)
DV-Xα计算机程序经过改进, 目前的计算能力为总原子数420个、 势类型数55个、 总电子数3000个[11, 12]。
在离散变分Xα原子簇计算中, 采用冻结芯轨道和自旋限制模型基函数取数值原子定位轨道(SSO)基, 即V为(1s2s2p)3s3p3d4s, H为1s2s (括号内的原子轨道为冻芯轨道), 外加半径为V: 6.0、 H: 4.0, 深为V: -1.5、 H: -1.0的势阱。 取样点分布采用Diophantus规则, 每个原子周围500个点, 自洽迭代的收敛精度为10~5。 本文用赝势法[13]来处理边界效应。
1.3 电子结构分析
要研究VHx(x=0, 1, 2)中电子结构和化学键的性质, 首先要研究它们的能级结构。 电子态密度可以用重叠高斯函数计算出来。 根据Mulliken集居数分析可以验证电子的重叠以及晶体中每个原子的带电情况。 材料的性能主要由化学键决定, 共价键和离子键是两种主要的化学键。 离子键在定性上和库仑引力相似, 即和原子所带电荷的乘积成正比, 和原子之间距离的平方成反比。 原子所带的净电荷是原子序数和原子的计算电子总布居之间的差值。 共价键的强度可以由原子之间的共价键级即占据的原子轨道之间的重叠布居来衡量。 共价键级由电子结构决定, 反映了原子之间电子云的成键重叠程度, 共价键级越大, 原子间共价键越强。 一般地说, A—B键的共价键级是A和B原子之间的重叠布居P(A—B), 其定义为[14]
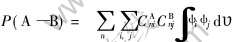
式中 ψi和ψj分别是A和B原子的第i和j个原子轨道, CAni和CBnj分别是该原子轨道在分子轨道中的组合系数, 对n的求和遍及所有电子占据的分子轨道。
2 结果与讨论
2.1 净电荷
晶簇中每个原子的带电情况如表1所列, 金属钒中每个原子所带电荷非常小, 都在零左右变化, 这与V作为金属单质的理论解释相吻合。 钒吸氢变为氢化物VH以后, V原子总是带正电, 而H原子总是带负电, 说明V相对于H是正电性元素; 氢化物VH中电荷的转移主要是从V原子转移到H原子, 由于电荷的转移导致它们离子间的相互作用, 所以VH中钒氢之间存在相互作用。 H原子与V原子之间的相互作用主要是由于它们之间电荷的转移所致, 转移的电荷数越多, 离子性相互作用越强。 V的净电荷在+0.32~+0.89范围之间, 与VH中V的化合价+1相比有一定的差值; H的净电荷在-0.44~-0.71之间, 与VH中H的化合价-1相比也有一定的差值。 表明VH中V—H键既存在离子性的相互作用, 又存在共价性的相互作用。 钒进一步吸氢变为氢化物VH2以后, 跟VH中一样, V原子总是带正电, 而氢原子总是带负电, 所以VH2中电荷的转移也是从V原子转移到H原子。 但在VH2中, V的净电荷在0.78~1.02之间, 与VH2中V的理论化合价+2相差较大。 H原子的净电荷只发生了微小的变化, 从-0.47~-0.71之间变化到了-0.50~-0.67。 即一部分H原子得到电子, 一部分H原子失去电子, 表明VH2中V—H键的相互作用既存在离子性的相互作用, 又存在共价性的相互作用。
2.2 Mulliken集居数
表1给出了V的3d、 4s轨道和H的1s、 2s轨道的Mulliken集居数, 可以看出, V+H→VH的过程中, V的4s轨道上的电子明显减少, H的1s轨道上的电子数明显增加, V的3d轨道和H的2s轨道上的集居数变化非常小。 说明了V的4s轨道上的电子向氢的1s轨道转移, 也就是说, 氢化物VH中, V—H之间的相互作用是由于V的4s轨道和H的1s轨道成键。 V进一步吸氢时, 即在VH+H→VH2过程中, V的3d轨道上的电子数都在减少。 V的4s轨道上的电子一部分减少, 另一部分却增加, 表明部分V的3d轨道和4s轨道发生了杂化。 H原子的1s轨道和2s轨道上的电子数没有明显的规律, 既有增加又有减少。 说明氢化物VH2中, V—H之间的相互作用是由于V的4s、 3d轨道和H的1s轨道之间成键。
2.3 能级结构和电子态密度
图2分别给出了V、 VH、 VH2的能级结构、 总电子态密度与H的1s、 V的3d、 4s轨道的分态密度。 从图中可以明显看出, 在V、 VH、 VH2中都是V的3d轨道的能量分布相对集中, 对总电子态密度的贡献最大, 而H的1s和V的4s却出现了能量分布非常分散的情况。 费米能级Ef位于V的3d区域。 在V、 VH、 VH2中都是Ef周围的态密度 最大, 说明它们较易得失电子。 图2(b)中, 轨道能
表1 VHx(x=0, 1, 2)中原子的净电荷和价轨道的电子集居数
Table 1 Net charge on each atoms and electron occupation numbers in
valence orbits of VHx(x=0, 1, 2)
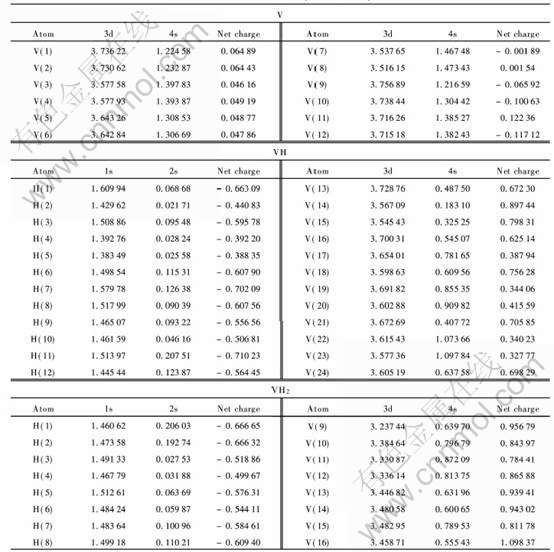
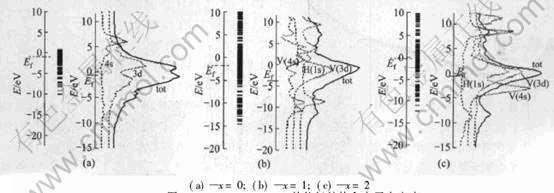
图2 VHx(x=0, 1, 2)的能级结构和电子态密度
Fig.2 Energy level structure and electron density of states for VHx(x=0, 1, 2)
量位于Ef以下的H的1s轨道和V的4s轨道部分重叠, 表明它们之间有成键作用; 位于Ef以上的1s轨道和V的4s轨道也有部分重叠, 表明它们之间又有反键作用。 从而说明VH材料中, V原子和H原子之间有着强烈的相互作用。 图2(c)中, 轨道能量位于Ef以下的H的1s轨道和V的3d轨道部分重叠, 说明H的1s轨道和V的3d轨道之间有成键作用; 位于Ef以上的H的1s轨道和V的4s轨道部分重叠, 说明1s轨道和V的4s轨道之间有反键作用。 这与前面的结论一致。 比较图2(b)和2(c)可见, 在VH+H→VH2的变化过程中, H的1s轨道的态密度明显地向高能量区域移动, 而V的3d和4s轨道的态密度稍微向低能量区域移动了一些。
因为Ef与氢化物的稳定性有密切的联系, Ef越低, 吸氢压越小, 氢化物稳定性越好[15]。 VH、 VH2的费米能级Ef计算结果分别为: -1.79eV、 -0.12eV。 即VH的费米能级比VH2的费米能级低, 所以VH比VH2更稳定。 因为Ef与氢化物的稳定性有密切的联系, Ef越低, 吸氢压越小, 氢化物稳定性越好[15]。 VH、 VH2的费米能级Ef 计算结果分别为: -1.79eV、 -0.12eV。 即VH的费米能级比VH2的费米能级低, 所以VH比VH2更稳定, VH2容易分解生成VH和H; 而V的费米能为-1.12eV, VH的费米能比V的费米能也低, VH再分解为V和H较难。 所以氢化物VH2中的氢不能完全被释放出来。
2.4 原子之间的键级
表2给出了“种子”(坐标圆点)原子(V原子)与周围所有不同类型的V原子、 H原子的键级。 在VH和VH2中, 两个V之间的键级总是负值或几乎接近于零, 说明V-V之间的共价性相互作用较小; 相反, H原子和V原子之间的键级很大而且都是正值, 这就表明V—H键之间是共价性的相互作用。 但是在VH中, V—H之间的键级不相等, V(13)—H(9)和V(13)—H(7)相差较大, 表明键级小的V(13)—H(7)所代表的V—H之间的相互作用更偏向离子性。 而在VH2中, V—H之间的键级都比VH中V—H之间的键级小, 表明它们之间的相互作用都偏向共价性, 并且V—H键的强度较弱, 所以VH2中的H容易释放出来。 这与前面净电荷的讨论和按鲍林的电负性理论解释是相吻合的。 因为V的电负性为1.6, 而H的电负性为2.1, 它们的电负性之差小于1.7, 所以V—H之间的相互作用偏向共价性。
2.5 电子密度差
为了进一步了解“种子”原子(V原子)与周围所有不同类型的V原子、 H原子的相互作用, 本文作者计算了V、 VH、 VH2中经过“种子”原子的(110)面的电荷密度差(图3)。 电荷密度差表示原子之间的电子重叠情况。 由图1可以看出, 在VH中, 一类氢原子与钒原子之间的电子云重叠较多, 说明V—H键之间主要是共价性相互作用; 一类氢原子 与钒原子之间的电子云重叠较少, 表明V—H键之间主要是离子性相互作用。 而在VH2中, 所有的氢原子与钒原子之间的电子云重叠都比较多, 表明V—H键之间的共价性相互作用较强, 这与前面的结论相吻合。
表2 计算的VHx(x=0, 1, 2)的共价键键级
Table 2 Calculated covalence levels of VHx(x=0, 1, 2)
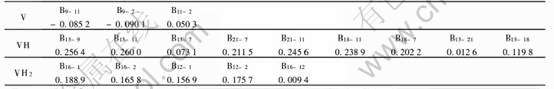
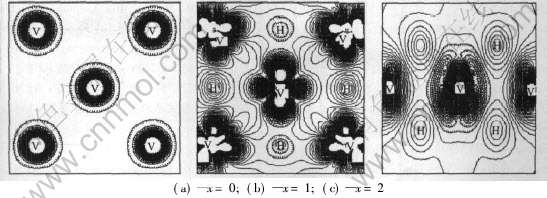
图3 VHx(x=0, 1, 2)中(110)面的电子密度差
Fig.3 Electron density difference of VHx(x=0, 1, 2) on plane (110)
3 结论
利用电荷自洽离散变分Xα(SCC-DV-Xα)方法计算了钒及其氢化物(VHx, x=0, 1, 2)的电子结构, 结果表明:1) 氢化物VH、 VH2中V—H键之间既有离子性又有共价性的相互作用; 2) VH2中V—H之间的键级比VH中V—H之间的键级小, 说明VH2中的H容易释放出来; 3) 氢化物VH中V的4s轨道和H的1s轨道作用成键; 氢化物VH2中V的4s、 3d轨道和H的1s轨道既有成键作用又有反键作用; 4) 氢化物VH比VH2的费米能级低, 说明VH更稳定。 通过以上计算, 解释了VH2中的氢不能全部放出的原因。
REFERENCES
[1]Budylkin N, Voloschin L, Mironova E, et al. Hydrogen isotopes mobility and trapping in V-Cr-Ti alloys[J]. Journal of Alloys and Compounds, 1996, 233-237: 1160-1162.
[2]Dug R, Nowicka E, Wolfram Z. Surface phenomena in the process of vanadium hydride formation[J]. Journal of Alloys and Compounds, 1997, 253-254: 496-499.
[3]Yamashita S, Komiya D, Yukawa K. Compositional dependence of phase stability of γ phase formed in vanadium alloys[J]. Journal of Alloys and Compounds, 2004, 364: 137-140.
[4]Buzlukov A L, Skripov A V. Nuclear magnetic resonance study of hydrogen motion in C15-type TaV2Hx (x≤ 0.18) [J]. Journal of Alloys and Compounds, 2004, 366: 61-66.
[5]Tsukahara M, Takahashi K, Isomura T. Influence of oxygen on hydrogen storage and electrode properties for micro-designed V-based battery alloys[J]. Journal of Alloys and Compounds, 1998, 265: 257-263.
[6]彭述明, 赵鹏骥. 金属钒及其氢化物的晶体结构模拟[J]. 原子能科学技术, 2000, 34(5): 469-472.
PENG Shu-ming, ZHAO Peng-ji. Simulation on crystal structures of metallic V and its hydrides[J]. Atomic Energy Science and Technology, 2000, 34(5): 469-472.
[7]Matumura T, Yukawa H, Morinaga M. Alloying effects on the electronic structures of VH2 and V2H[J]. Journal of Alloys and Compounds, 1999, 284: 82-88.
[8]Yukawa H, Matsumura T, Morinaga M, et al. Chemical bond state and hydride stability of hydrogen storage alloys[J]. Journal of Alloys and Compounds, 1999, 293-295: 227-230.
[9]Yukawa H, Takagi M, Teshima A, et al. Alloying effects on the stability of vanadium hydrides[J]. Journal of Alloys and Compounds, 2002, 330-332: 105-109.
[10]童宏勇, 顾牡, 汤学峰. PbWO4电子结构的密度泛函计算[J]. 物理学报, 2000, 49(8): 1545-1548.
TONG Hong-yong, GU Mu, TANG Xue-feng. Theoretic calculation on the electronic structure of PbWO4 crystals[J]. Acta Phys, 2000, 49(8): 1545-1548.
[11]李荣. 锂离子电池锰系正极材料电子结构的量子化学研究[D]. 重庆: 重庆大学, 2002.
LI Rong. The Quantum Chemical DV-Xα Study on the Electronic Structure of Lithium-Manganese-Oxides Electrode Material for Lithium Ion Battery[D]. Chongqing: Chongqing University, 2002.
[12]李荣, 陈昌国, 梁国明, 等. 锂离子电池正极材料LixMn2O4电子结构的量子化学DV-Xα研究[J]. 中国有色金属学报, 2004, 14(5): 865-870.
LI Rong, CHEN Chang-guo, LIANG Guo-ming, et al. Quantum chemical DV-Xα study on electronic structure of electrode material LixMn2O4 for lithium ion battery[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(5): 865-870.
[13]潘毓刚, 李俊清, 祝继康. Xα方法的理论和应用[M]. 北京: 科学出版社, 1987.
PAN Yu-gang, LI Jun-qing, ZHU Ji-kang. Theory and Practice of Discrete Variational Method[M]. Beijing: Science Press, 1987.
[14]赵世玺, 闵新民, 刘韩星, 等. S-M(M=Al, Co)复合掺杂的LiMn2O4结构稳定性[J]. 物理化学学报, 2004, 20(3): 233-236.
ZHAO Shi-xi, MIN Xin-min, LIU Han-xing, et al. The structure stability of S-M(M=Al, Co) co-doped spinel LiMn2O4 cathode materials[J]. Acta Phys Chim Sin, 2004, 20(3): 233-236.
[15]陈宁, 林勤, 叶文, 等. RENi5电子结构与吸氢性能关系研究[J]. 科学通报, 1995, 40(24): 2234-2236.
CHEN Ning, LIN Qing, YE Wen, et al. Relationship of electronic structure of RENi5 and hydrogen storage properties[J]. Chin Sci Bulletin, 1995, 40(24): 2234-2236.
(编辑袁赛前)
基金项目: 重庆市自然科学基金资助项目(CSTC2004BB4169); 重庆师范大学博士科研基金资助项目
收稿日期: 2004-07-20; 修订日期: 2004-12-13
作者简介: 李 荣(1970-), 男, 讲师, 博士.
通讯作者: 李 荣, 博士; 电话: 023-65362702; E-mail: Rongli258@hotmail.com