文章编号:1004-0609(2008)05-0873-06
Mg-Mg2Ni1-xMex的氢化反应动力学
赵显久1,李 谦1,林根文1,周国治1, 2,张捷宇1,鲁雄刚1
(1. 上海大学 材料科学与工程学院,上海 200072;
2. 北京科技大学 冶金与生态工程学院,北京 100083)
摘 要:利用新动力学模型分析了第三组元Co和Fe取代Ni后对Mg-Mg2Ni吸氢反应动力学的影响规律,探讨其吸氢反应的控速环节,并求解出活化能,同时验证该新模型同样适用于该体系的动力学研究。结果表明:Mg-Mg2Ni1-xMex (Me=Co, Fe; x=0, 0.1, 0.3)氢化反应的控速环节是氢在氢化物层的扩散步骤,Co和Fe取代Ni后对Mg-Mg2Ni的氢化反应速率有影响,但未改变其吸氢反应动力学机制,理论预报值与实验实测值较符合;对于相同吸氢条件,同等取代量的Mg-Mg2Ni1-xCox的特征吸氢时间比 Mg-Mg2Ni1-xFex的低,说明Co比Fe取代Ni更有利于提高材料的氢化反应速率。
关键词:Mg-Mg2Ni1-xMex;储氢合金;氢化反应;动力学
中图分类号:TG 139.7 文献标识码:A
Hydriding reaction kinetics of Mg-Mg2Ni1-xMex compositions
ZHAO Xian-jiu1, LI Qian1, LIN Gen-wen1, CHOU Kuo-chih1, 2, ZHANG Jie-yu1, LU Xiong-gang1
(1. School of Materials Science and Engineering, Shanghai University, Shanghai 200072, China;
2. School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: A new kinetics model proposed was applied to investigate the effect of additives (Co and Fe) on the hydriding kinetic properties of composites based on Mg and Mg2Ni. Much attention was paid to discuss the rate-controlled step, and the reaction activation energy was calculated. The results show that this model is validated by comparison of these calculation results with experimental data. Its kinetic mechanism was well established and its rate-limited step in the hydriding reaction of Mg-Mg2Ni1-xMex composite (Me=Co, Fe; x=0, 0.1, 0.3) is hydrogen diffusion in the metal hydride, which means the additives (Co and Fe) can impose on the hydriding rate without changing the kinetic mechanism of the hydriding reaction. Under the same conditions for hydrogen absorption, the same amount of the additive Co is more profitable to promote the hydriding rate of the composites based on Mg and Mg2Ni than the same amount of the additive Fe on account of the hydriding characteristic time, and the activation energy of the former is smaller than that of the latter.
Key words: Mg-Mg2Ni1-xMex; hydrogen storage alloys; hydriding reaction; kinetic
在推进镁基储氢合金的实用进程中,针对其热力学和动力学的改性研究一直是人们关注的热点
[1-2]。迄今为止,大多数研究主要集中在镁基储氢合金的热力学问题上
[3-4]。元素取代法是改善镁基合金性能的一种行之有效的方法。在Mg
2Ni合金中添加第三组元Co
[5],La
[6]和Ag
[7]等,在一定程度上改善了Mg
2Ni的充放氢性能。对镁基储氢合金的动力学研究中,理论研究滞后于实验研究
[8-9]。影响镁基合金氢化反应动力学的因素很多,若全面考察,数学的求解过程复杂
[10-11],难以进行理论分析和讨论。本课题组近年来提出一个描述气固相反应动力学的新模型,并应用到储氢合金的吸放氢反应、陶瓷材料的氧化反应和含碳球团矿还原反应的动力学研究中
[12-13]。本文作者采用该新模型研究第三组元Co和Fe对Mg-Mg
2Ni
1-xM
x (M=Co,Fe;x=0.1,0.3)吸氢反应动力学性能的影响规律,探讨其氢化反应的动力学机理。
1 氢化反应动力学模型
合金的氢化反应可大致分为如下4个阶段:1) 氢分子被吸附在金属表面并分解为氢原子;2) 被吸附的氢原子向金属体内扩散,固溶于金属中形成金属固 溶相;3) 固溶相进一步与氢反应,生成氢化物相层; 4) 氢化物层剥落或氢通过氢化物层进一步扩散。氢化物层剥落后,氢与袒露的新鲜金属表面进一步进行如上4个阶段的反应。若氢化物不剥落,氢则通过氢化物层扩散进入到金属体内发生反应。当氢通过氢化物层的扩散过程为反应速率的控制步骤时,其反应速度遵循抛物线状速度定律[14]:m = At1/2(式中m为金属表面单位面积上生成的氢化物质量,A为常数,t为时间)。这表明金属表面单位面积上生成的氢化物层质量m与时间t的平方根成正比,但当氢化物以薄片形式脱落时,氢化物的质量仅取决于氢在合金表面的反应速度,为时间t的一次函数。
借鉴LIN等[15]利用不同的动力学模型研究储氢合金MLNi2.8(Co, Mn, Al)1.2的α-β相区动力学机理的方法,本课题组也曾逐一运用41个方程式对Mg2Ni[16],Mg-Ag-Ni[17]和Mg-Al-Ni[18]吸放氢动力学进行研 究,但过程比较繁琐,而且所得数值解不便于分析讨论。基于此,本文作者拓展了储氢合金动力学理论的研究[12, 19]。
新的动力学模型建立在如下的前提假设上:吸氢颗粒为均匀的等直径等密度的圆球;认为整个反应步骤中,氢在氢化物中的扩散速度比其他步骤的扩散速度慢,是控制整个反应的限制性环节。由于反应分数和半径的立方成正比,氢在氢化物层中的扩散流量与半径/时间的变化率成正比。根据Fick第一定律,得到反应分数与时间的微分关系式:

对式(1)积分得到反应分数与时间关系式:

令 ,则式(2)的表现形式和Jander[20]公式[1-(1-ξ)1/3]2 = kt是一样的。Jander模型本质上是表征扩散为控速环节的反应动力学过程,这说明新模型的前提假设是合理的。在新模型中,因子k得到显化,给出影响k的本质因素。将物质的特征吸附时间定义为储氢合金吸氢达到一定量时所需要的时间,由式(2)可得:
,则式(2)的表现形式和Jander[20]公式[1-(1-ξ)1/3]2 = kt是一样的。Jander模型本质上是表征扩散为控速环节的反应动力学过程,这说明新模型的前提假设是合理的。在新模型中,因子k得到显化,给出影响k的本质因素。将物质的特征吸附时间定义为储氢合金吸氢达到一定量时所需要的时间,由式(2)可得:
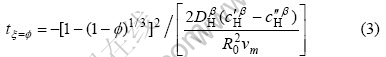
结合式(2)和(3),得到ξ—t的函数关系:

温度的变化直接影响物质扩散过程,进而影响吸氢反应速率。众所周知,温度对扩散系数的影响服从Arrhenius公式,将Arrhenius公式代入式(3)得:

将2个不同温度下同一时间对应的反应分数代入式(5),消除相同项后再代入式(4)得:
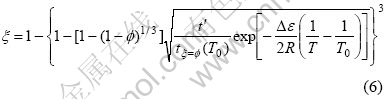
求取材料的吸氢活化能时,选择同一反应时间,测量2个不同温度T′和T″下的ξ′和ξ″,结合最小二乘法,根据式(6),消去t, 得:
得:

利用式(4)~(7),根据实验数据可分别计算某一温度下的反应分数和时间的函数关系,量化反应的活化能和预报未知温度下的动力学曲线。
氢气分压也是影响合金氢化反应的重要因素之一,与气相平衡的β相中氢浓度(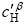 )可用气相中的氢分压表示为
)可用气相中的氢分压表示为

将式(8)代入式(3)得:

结合式(9)和不同氢分压对应的特征时间,经过数学处理得:
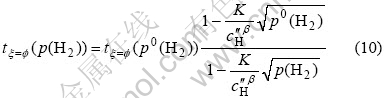
将式(10)代入式(4)得:
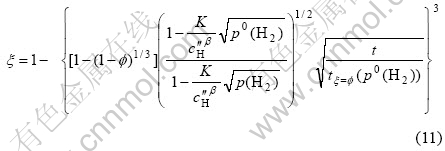
式(11)表明,ξ随氢压的增高而增加。
在实际处理中,可将反应分数与最大储氢量相乘,得到相应的时间与绝对吸氢量的关系式。
2 结果和讨论
2.1 Mg-Mg2Ni1-xCox的氢化反应动力学
GRIGOROVA等[4]研究了Mg-Mg2Ni1-xMex (Me = Fe, Co; x = 0, 0.1, 0.3),Mg-Mg2Ni-V和Mg-Mg2Ni-C系列储氢材料的吸放氢性能,结果表明,Mg-15%(质量分数)Mg2Ni0.9Co0.1在423 K和1 MPa H2下吸氢超过4%,改性效果最明显。KHRUSSANOVA等[5]采用机械合金化制备得到Mg-Mg2Ni1-xCox,通过研究发现,该体系比通常的镁基储氢合金具有更高的吸放氢容量和更快的吸放氢速度。但是,他们仅仅从实验角度来表征其结果,而未对其吸放氢动力学机理从理论上进行分析和讨论。
结合文献[4]中Mg-Mg2Ni1-xCox (x = 0, 0.1, 0.3)在473 K和1 MPa条件下吸氢速度实验数据,包括某一反应分数下对应的特征吸附时间,分别代入式(4)进行理论计算后并绘制于图1。由图1可知,理论计算值与实验值较吻合。根据新模型成立的前提条件,可以认为Mg-Mg2Ni1-xCox (x = 0, 0.1, 0.3)在473 K和1 MPa条件下吸氢过程的限制性环节为氢在氢化物层中的扩散。量化结果显示,Co取代Ni对提高材料的氢化反应速度有一定效果,但没有改变其氢化反应动力学机制。当Co添加量为0.1时,对Mg-Mg2Ni吸氢速度改性效果更好。
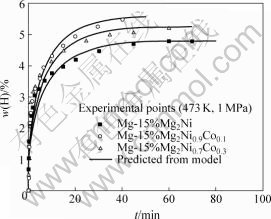
图1 473 K和1 MPa条件下Mg-Mg2Ni1-xCox (x = 0, 0.1, 0.3)的氢化反应动力学实验值和计算值的对比
Fig.1 Comparison of kinetic curves between experimental data and theoretical prediction of hydriding reaction of Mg-Mg2Ni1-xCox (x = 0, 0.1, 0.3) at 473 K and 1 MPa
利用KHRUSSANOVA等[5]的实验数据点并结合式(7),可以求得Mg-15%Mg2Ni0.9Co0.1的吸氢反应活化能,其表达式为

将计算值代入式(6),可以得出Mg-15%Mg2Ni0.9- Co0.1在其他温度下的吸氢反应动力学曲线,计算公式如下(ξ1代表氢化反应分数):

分别将拟报的不同温度T代入式(13),计算出各温度下的吸氢速率并绘于图2(a)中。由图2(a)可知,预测曲线与实验数据较吻合。此外,还可利用式(13)计算Mg-15%Mg2Ni0.9Co0.1在323,393和403 K的吸氢反应动力学曲线,这是文献中没有进行的实验温度,利用模型同样可以进行理论计算。

图2 在323~573 K和1 MPa条件下Mg-15%Mg2Ni0.9Co0.1(a)和Mg-15%Mg2Ni0.7Co0.3(b)的氢化反应动力学实验值和计算值的对比
Fig.2 Comparison of kinetic curves between experimental data and theoretical prediction of hydriding reaction of Mg- 15%Mg2Ni0.9Co0.1(a) and Mg-15%Mg2Ni0.7Co0.3(b) at 323- 573 K and 1 Mpa
同理,对Mg-15%Mg2Ni0.7Co0.3氢化反应动力学进行理论计算,并与实验值绘制于图2(b),求得其氢化反应的活化能为69.12 kJ/mol,它比Mg-15%Mg2Ni0.9- Co0.1的氢化反应活化能大。从图1和图2所示的计算结果可知,随着Co添加量x从0.1变化到0.3,材料的特征吸氢时间延长,其氢化反应的活化能变大,说明其反应速度变慢。
2.2 Mg-Mg2Ni1-xFex的氢化反应动力学
GRIGOROVA等[4]运用高能球磨制备了Mg- 15%Mg2Ni1-xFex (x=0,0.1,0.3),并研究其吸放氢属性。结果表明,该材料不需活化即可达到较高的储氢容量,降低吸放氢温度,加快吸放氢速度。将该文献的实验数据整理后代入式(4),所得理论计算值与实验值见图3。由图3可知,预测曲线与实验数据较吻合。
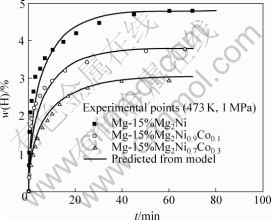
图3 473 K和1 MPa条件下Mg-Mg2Ni1-xFex (x = 0, 0.1, 0.3)的氢化反应动力学实验值和计算值的对比
Fig.3 Comparison of kinetic curves between experimental data and theoretical prediction of hydriding reaction of Mg-Mg2Ni1-xFex (x = 0, 0.1, 0.3) at 473 K and 1 MPa
结合BOBET等[21]的部分实验数据和式(7),可以求得Mg-15%Mg2Ni0.9Fe0.1的吸氢活化能,其表达式为

将活化能计算值代入式(6)(ξ2代表氢化反应分数),可以得出Mg-15%Mg2Ni0.9Fe0.1在其他温度下的吸氢动力学曲线。
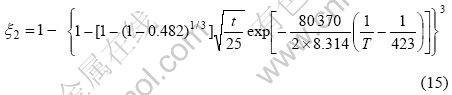
分别将拟预报的不同温度T代入式(15),计算出各温度下的吸氢速率,结果如图4(a)所示。由图4(a)可知,预测值与实验值较吻合。此外,还可利用式(15)得出Mg-15%Mg2Ni0.9Fe0.1在403 K温度下的吸氢动力学曲线,这是文献中没有进行的实验温度。
同理,对Mg-15%Mg2Ni0.7Fe0.3氢化反应动力学进行理论计算,并与实验值绘制于图4(b),求得反应的活化能为83.57 kJ/mol,它比Mg-15%Mg2Ni0.9Fe0.1的氢化反应活化能大。从图3和图4所示的计算结果可知,随着Fe添加量x从0.1变为0.3,材料的特征吸氢时间变长,其氢化反应的活化能变大,说明其反应速度变慢。
2.3 Mg-Mg2Ni1-xMex的氢化反应动力学
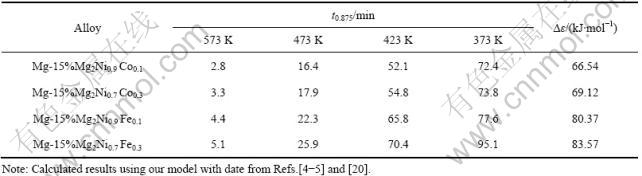
利用新模型所得计算结果可知,Co和Fe取代Ni后,对Mg-Mg2Ni材料的吸氢速度有不同程度的影响。表1所列为各种材料在373~573 K和1 MPa条件下的特征吸氢时间和反应活化能。由表1可知,在同样的吸氢条件下,Co和Fe的添加量x从0.1变化到0.3时,其特征时间增大,活化能变大,其吸氢速率减小;说明Co和Fe取代Ni的量只有适量,才有利于其吸氢反应动力学性能的改善。对于相同添加量的Co和Fe,Mg-Mg2Ni1-xCox (x = 0.1, 0.3)的特征吸氢时间比Mg-Mg2Ni1-xFex (x = 0.1, 0.3)的小,而且前者的活化能也小于后者,说明等量的Co对Mg-Mg2Ni吸氢性能的改善效果较Fe的好。从上面的对比分析可知,材料的“特征吸氢时间”可以直接衡量材料氢化反应速度,具有“指针”作用,一般是特征吸附时间越短,吸氢速度越快。从图1~4可知,模型理论计算值和实验实测值较吻合,说明新模型在Mg-Mg2Ni1-xMex (Me = Co, Fe; x = 0, 0.1, 0.3)吸氢过程动力学的应用是可行的,同时证明Mg-Mg2Ni1-xMex (Me = Co, Fe; x = 0, 0.1, 0.3)氢化反应的限制性环节是氢的扩散步骤,Co和Fe取代Ni后,仅仅改变了Mg-Mg2Ni的吸氢速度,但是没有改变其动力学机制。
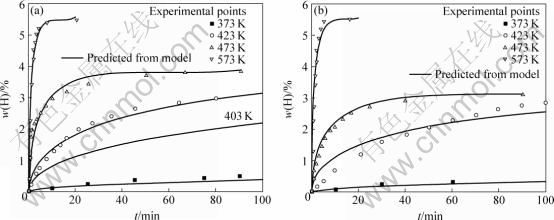
图4 在373~573 K和1 MPa条件下Mg-15%Mg2Ni0.9Fe0.1(a)和Mg-15%Mg2Ni0.7Fe0.3(b)的氢化反应动力学实验值和计算值对比
Fig.4 Comparison of kinetic curves between experimental data and theoretical prediction of hydriding reaction of composites Mg-15%Mg2Ni0.9Fe0.1(a) and Mg-15%Mg2Ni0.7- Fe0.3(b) at 373-573 K and 1 MPa
表1 373~573 K 和1 MPa下Mg-Mg2Ni1-xMex (Me = Co, Fe; x = 0.1, 0.3)氢化反应的特征时间和活化能的对比
Table 1 Comparison of hydriding characteristics time (t0.875) and activation energies for Mg-Mg2Ni1-xMex at 373-573 K and 1 MPa
3 结论
1) 可以将新动力学模型应用到Mg-Mg2Ni1-xMx (M=Co, Fe; x=0, 0.1, 0.3)氢化反应动力学研究中,该材料吸氢过程的控速步骤为氢在氢化物层的扩散。
2) Co和Fe取代Mg-Mg2Ni中的Ni后,改变了合金的吸氢速率,但没有改变其原有的吸氢反应动力学机制。
3) 各材料特征吸氢时间表明,相同量的Co取代Ni对Mg-Mg2Ni吸氢性能的改善效果明显比等量Fe取代Ni的合金的效果好。相同吸氢条件下,Mg- 15%Mg2Ni0.9Co0.1的特征吸氢时间最短,氢化反应速率最快,其活化能为66.54 kJ/mol。
REFERENCES
[1] 胡子龙. 贮氢材料[M]. 北京: 化学工业出版社, 2002.
HU Zi-long. Storage material[M]. Beijing: Chemical Industry Press, 2002.
[2] MARTIN M, GOMMEL C, BORKHART C, FROMM E. Absorption and desorption kinetics of hydrogen storage alloys[J]. J Alloys Comp, 1996, 238: 193-201.
[3] 王秀丽, 涂江平, 张孝彬, 荣 伟, 陈长聘. 纳米晶Mg2-xTixNi0.8Cr0.2四元合金的气态储氢性能[J]. 中国有色金属学报, 2004, 14(6): 1020-1024.
WANG Xiu-li, TU Jiang-ping, ZHANG Xiao-bin, RONG Wei, CHEN Chang-pin. Hydrogen storage properties of tetragonal nanocrystalline Mg2-xTixNi0.8Cr0.2 alloy[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 1020-1024.
[4] GRIGOROVA E, KHRISTOV M, KHRUSSANOVA M, BOBET J L, PESHEV P. Effect of additives on the hydrogen sorption properties of mechanically alloyed composites based on Mg and Mg2Ni[J]. Int J Hydrogen Energy, 2005, 30: 1099-1105.
[5] KHRUSSANOVA M, GRIOVA E, BOBET J L, KHRISTOV M, PESHEV P. Hydrogen sorption properties of nanocomposites Mg-Mg2Ni1-xCox obtained by mechanical alloying[J]. J Alloys Comp, 2004, 365: 308-313.
[6] LI Qian, CHOU Kou-chih, XU Kuang-di, LIN Qin, JIANG Li-jun, ZHAN Feng. Determination and interpretation of hydriding and dehydriding kinetics in mechanically alloyed LaNiMg17 composite[J]. J Alloys Comp, 2004, 14(6): 1020-1024.
[7] 房文斌, 张文丛, 于振兴, 王尔德. 镁基储氢材料的研究进展[J]. 中国有色金属学报, 2002, 12(5) : 853-862.
FANG Wen-bin, ZHANG Wen-cong, WANG Er-de. Recent development of Mg-based hydrogen storage material[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(5): 853-862.
[8] BLOCH J, MINTZ M H. Kinetics and mechanisms of metal hydrides formation—A review[J]. J Alloys Comp, 1997, 253/254: 529-541.
[9] ASAKUMA Y, MIYANCHI S, YAMAMOTO T. Numerical analysis of absorbing and desorbing mechanism for the metal hydride by homogenization method[J]. Int J Hydrogen Energy, 2003, 28: 529-536.
[10] MINTZ M H, ZEIRI Y. Hydriding kinetics of powders[J]. J Alloys Comp, 1994, 216: 159-175.
[11] BLOCH J. The kinetics of a moving metal hydride layer[J]. J Alloys Comp, 2000, 312: 135-153.
[12] CHOU Kou-chih, LI Qian, LIN Qin, JIANG Li-jun. Kinetics of absorption and desorption of hydrogen in alloys powder[J]. Int J Hydrogen Energy, 2005, 30: 301-309.
[13] CHOU Kou-chih. A kinetic model for oxidation of Si-Al-O-N materials[J]. J American Ceramic Society, 2006, 89(5): 1568-1576.
[14] YASUAKI O S. 金属氢化物的性质与应用[M]. 吴永宽, 苗艳秋, 译. 北京: 化学工业出版社, 1990.
YASUAKI O S. Application and properties of metal hydrides[M]. WU Yong-kuan, MIAO Yan-qiu, transl. Beijing: Chemical Industry Press, 1990.
[15] LIN Qin, CHEN Ning, YE Wen. Kinetics of hydrogen absorption in hydrogen storage alloy[J]. J Univ Sci Technol Beijing, 1997, 4(2): 34-37.
[16] LI Qian, LIN Qin, JIANG Li-jun, CHOU Kuo-chih, ZHAN Feng, ZHENG Qiang. Characteristics of hydrogen storage alloy Mg2Ni produced by hydriding combustion synthesis[J]. J Mater Sci Technol, 2004, 20(2): 209-212.
[17] LI Qian, CHOU Kou-chih, LIN Qin, JIANG Li-jun, ZHAN Feng. Hydrogen absorption and desorption kinetics of Ag-Mg-Ni alloys[J]. Int J Hydrogen Energy, 2004, 29: 843-849.
[18] LI Qian, LIN Qin, CHOU Kuo-chih, JIANG Li-jun, ZHAN Feng. A study on the hydriding-dehydriding kinetics of Mg1.9Al0.1Ni[J]. J Materials Science, 2004, 39: 61-65.
[19] CHOU Kou-chih, XU Kuang-di. A new model for hydriding and dehydriding reactions in intermetallics[J]. Intermetallics, 2007, 15: 767-777.
[20] JANDER W. Reactions in the solid state at high temperatures[J]. Z Anorg Allgem Chem, 1927, 163(1/2): 1-30.
[21] BOBET J L, GRIGOROVA E, KHRUSSANOVA M, KHRISTOV M, RADEV D, PESHEV P. Hydrogen sorption properties of the nanocomposites Mg-Mg2Ni1-xFex[J]. J Alloys Comp, 2002, 345: 280-285.
基金项目:全国博士学位论文作者专项资金资助项目(200746);上海市教育委员会科研基金资助项目(06AZ001);上海市基础研究重点资助项目(06JC14031);上海市青年科技启明星计划资助项目(06QA14021)
收稿日期:2007-02-27;修订日期:2008-01-16
通讯作者:李 谦,副研究员,博士;电话/传真:021-56338065;E-mail: shuliqian@shu.edu.cn
(编辑 龙怀中)