
Isolation and identification of moderately thermophilic acidophilic iron-oxidizing bacterium and its bioleaching characterization
ZENG Wei-min(曾伟民)1, 2, WU Chang-bin(邬长斌)1, ZHANG Ru-bing(张汝兵)1,
HU Pei-lei(胡培磊)1, QIU Guan-zhou(邱冠周)1, 2, GU Guo-hua(顾帼华)1, 2, ZHOU Hong-bo(周洪波)1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy, Ministry of Education, Central South University, Changsha 410083, China
Received 28 February 2008; accepted 1 July 2008
Abstract: A moderately thermophilic acidophilic iron-oxidizing bacterium ZW-1 was isolated from Dexing mine, Jiangxi Province, China. The morphological, biochemical and physiological characteristics, 16S rRNA sequence and bioleaching characterization of strain ZW-1 were studied. The optimum growth temperature is 48 ℃, and the optimum initial pH is 1.9. The strain can grow autotrophically by using ferrous iron or elemental sulfur as sole energy sources. The strain is also able to grow heterotrophically by using peptone and yeast extract powder, but not glucose. The cell density of strain ZW-1 can reach up to 1.02×108 /mL with addition of 0.4 g/L peptone. A phylogenetic tree was constructed by comparing with the published 16S rRNA sequences of the relative bacteria species. In the phylogenetic tree, strain ZW-1 is closely relative to Sulfobacilus acidophilus with more than 99% sequence similarity. The results of bioleaching experiments indicate that the strain could oxidize Fe2+ efficiently, and the maximum oxidizing rate is 0.295 g/(L·h). It could tolerate high concentration of Fe3+ and Cu2+ (35 g/L and 25 g/L, respectively). After 20 d, 44.6% of copper is extracted from chalcopyrite by using strain ZW-1 as inocula.
Key words: moderately thermophilic acidophilic bacterium; biochemical and physiological characteristics; phylogenetic analysis; chalcopyrite
1 Introduction
The resource of high grade copper ores in the world is becoming more and more scarce, and the low grade copper ores cannot be treated economically and effectively by conventional mineral processing, such as pyrometallurgical refining, which always causes serious pollution to environments. Therefore, a lot of efforts have been made in the development of biohydrometallurgical process for these ores. Among these biohydrometallurgical processes, bioleaching of primary sulphide ores (chalcopyrite) with moderately thermophilic microorganisms has been paid more attention. Using thermophiles (compared with mesophiles) to leach sulphide ores not only highly improves the reaction kinetics, but also avoids excessive passivation of chalcopyrite that hinders the durative bioleaching[1-4]. The majorities of extreme thermophiles surviving above 60 ℃ belong to Archaea, which always lacks cellwall and can not survive in high pulp density. At present, the application of extreme thermophiles in industry is difficult. However, moderately thermophilic microorganisms can tolerate higher pulp density than extreme thermophilic microorganisms[5], so they have the advantage of application in bioleaching of chalcopyrite.
Currently, the moderately thermophilic micro- organisms mainly include Acidithiobacillus caldus, Leptospirillum ferriphilum, Acidimicrobium ferrooxidans, Sulfobacillus thermosulfidooxidans and Sulfobacillus acidophilus. They can grow autotrophically by oxidizing ferrous iron, sulphur, and/or sulphide ores. Their optimum growing temperature and pH value are 45-60 ℃ and 1.3-2.5, respectively.
There are several semi-industrial trials of chalcopyrite bioleaching with moderately thermophilic microorganisms overseas[6-7]. In China only a few studies involve in the application of moderately thermophilic microorganisms in bioleaching. In this work, a moderately thermophilic acidophilic iron- oxidizing bacterium was isolated from Dexing mine, Jiangxi Province, China, and its identification and bioleaching characterization was carried out to provide some evidence for its application in industry.
2 Materials and methods
2.1 Enrichment of acidophilic moderately thermo- philic bacteria
The acid mine drainage samples were collected from Dexing mine, Jiangxi Province, China. The medium used for enrichment consisted of the following compounds: (NH4)2SO4 3.0 g/L, Na2SO4 2.1 g/L, MgSO4?7H2O 0.5 g/L, K2HPO4 0.05 g/L, KCl 0.1 g/L and Ca(NO3)2 0.01 g/L. 30 g/L FeSO4·7H2O was added as energy source. The samples were enriched at 48 ℃ and initial pH of 2.0.
2.2 Isolation of bacteria and its morphological observation
Isolation of bacteria was carried out through several serial dilutions of above mentioned enrichment culture in test tube. When the morphology of enrichment appeared to be homologous (observed with the optical microscope), pure culture was considered to be obtained. Pure culture (named as strain ZW-1) was incubated in rotary shakers under 180 r/min at 48 ℃ and initial pH of 2.0. After being cultured for 3 d, samples were collected for Gram’s stain and SEM observation.
2.3 Biochemical and physiological characteristics
2.3.1 Optimization of temperature and initial pH for growth
The optimum temperature for growth was obtained by determining bacterial growth in liquid medium at different temperatures, i.e., 39, 42, 45, 48, 51 and 54 ℃. And optimum pH for bacterial growth was determined at pH values ranging from 0.6 to 3.4. The growth was compared by estimating the amount of biomass in different conditions.
2.3.2 Effect of organic compound on bacterial growth
Three different concentrations of peptone, yeast extract powder and glucose (0, 0.2, 0.4, 0.6 and 0.8 g/L) were added into culture medium, respectively. Strain ZW-1 at stable growth stage was inoculated with 5% inoculated amount, at pH 2.0 and 48 ℃. The bacterial growth was compared also by estimating the amount of biomass under different conditions.
2.3.3 Utilization of substrates
In this experiment, S0, S0+yeast extract powder (0.4 g/L), yeast extract powder (0.4 g/L), peptone (0.4 g/L), glucose (0.4 g/L), pyrite (5 g/L) and chalcopyrite (5 g/L) were respectively added into culture medium as the energy source, instead of FeSO4·7H2O. Strain ZW-1 at stable growth stage was inoculated with 5% inoculated amount, and the incubation was performed at pH 2.0 and 48 ℃.
2.4 PCR amplification, sequencing and phylogenetic analysis of 16S rRNA
DNA was extracted from the 25 mL inoculated vessels by centrifuging at 12 000 r/min for 10 min to pellet microorganisms. Then the pallet was washed with TE (pH 8.0) three times. After 6 ?L 1% proteinase K solution and 100 ?L 10% SDS were added, the mixture was incubated at 37 ℃ for 1-4 h. Subsequently, one or two extraction steps were performed by adding equal volumes of Tris-hydroxybenzene until there is no protein between organic and liquid phases. Then, the same volume of chloroform was used to extract the DNA samples. The genomic DNA was precipitated by adding 2-3 times volumes of alcohol to the samples. To ensure a complete precipitation, the mixture was stored at -20 ℃ for 15-20 min. The precipitated DNA was recovered by centrifugation at 12 000 r/min for 10 min, re- suspended in TE buffer (pH 8.0) and stored at -20 ℃. DNA preparations were separated by electrophoresis in a 1% agar gel in tris-acetate-EDTA buffer and quantified visually under UV light through staining with ethidium bromide (EB) and compared with standards of known length.
After the extraction of DNA, PCR amplification was performed. A portion of the bacterial 16S rRNA gene was amplified using the primers, 27F (5’- AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’- TACCTTGTTACGACTT-3’). The reaction mixture was set up on ice as follows: 10 ng template DNA, 1×PCR buffer, 1.5 mmol/L MgCl2, 0.2 ?mol/L forward primer, 0.2 ?mol/L reverse primer, 0.2 mmol/L of each dNTP, 0.8U Taq-DNA-Polymerase, and adding bi-distilled H2O to 50 μL final volume. All chemicals and enzymes were provided by Fermentas. The PCR program was 94 ℃, 4 min, followed by 30 cycles of 94 ℃, 45 s; 55 ℃, 45 s; and 72 ℃, 90 s and finally 72 ℃, 10 min. When the PCR program finished, the PCR product was separated by gel electrophoresis on a 1% agar gel in tris/acetate-buffer and analyzed by staining with ethidium bromide(EB) under UV light. The band of the expected size (approximately 1 460 bp) was cut off and purified with a commercial kit (Gel Extraction Kit, Promega, USA). The expected bands were sequenced by Sunbiotech Company (Beijing, China). Sequence identification was initially estimated by using the BLASTN facility of the National Center for Biotechnology Information (http://www.ncbi. nlm.nih.gov/blast/). All available subsets of 16S rRNA gene sequences were selected, analyzed and aligned with CLUSTALX 1.8, and the final phylogenetic tree was generated by MEGA 4.0.
2.5 Bioleaching experiments
Experiments to study the ability of strain ZW-1 to oxidize Fe2+, tolerate Fe3+ and Cu2+ and bioleach chalcopyrite were conducted in a 500 mL shake flask with 200 mL medium at 48 ℃ and pH 2.0. In the Fe2+ oxidation experiment, 30 g/L FeSO4·7H2O was added into the medium (with 6.08 g/L Fe2+). The concentration of Fe2+ in the solution was measured every 12 h at the first stage and the last stage, and every 6 h in the middle stage. In the experiments to investigate the tolerence of strain ZW-1 to Fe3+ and Cu2+, Fe2(SO4)3 and CuSO4·5H2O were added until the concentration of Fe3+ and Cu2+ achieved 5, 10, 15, 20, 25, 30 and 35 g/L, respectively. The amount of bacterium was counted with the optical macroscope after being cultured for 3 d. In the chalcopyrite bioleaching experiment, 12 g/L chalcopyrite (with diameter less than 75 μm) was added as energy source instead of FeSO4·7H2O, and the components of chalcopyrite are listed as Table 1. The concentrations of Fe3+ and Cu2+ were analyzed every day. The acid consumption was compensated by 10 mol/L sulfuric acid to keep pH value around 2 at the first stage. Distilled water was added in order to compensate for evaporation losses.
Table 1 Components of chalcopyrite (mass fraction, %)

In the bioleaching experiments, the components of ore sample were analyzed by XRD. Copper and total iron concentrations in solution were determined by atomic absorption spectrophotometer. Ferrous iron concentration in solution was assayed by titration with potassium dichromate. The pH value was measured with pH S-3C acid meter.
3 Results and discussion
3.1 Isolation and morphological observation
A moderately thermophilic acidophilic iron- oxidizing bacterium named strain ZW-1 was isolated from Dexing mine, Jiangxi Province, China. It is gram- negative and long rod-shaped. The cell of strain ZW-1 is 0.8-1.5 μm in length and about 0.4 μm in diameter. The morphological characteristic is shown in Fig.1.
3.2 Biochemical and physiological characteristics
3.2.1 Optimal temperature for growth
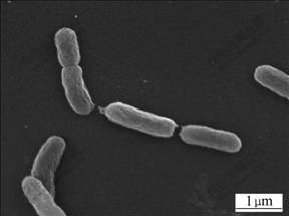
Fig.1 SEM image of strain ZW-1
When strain ZW-1 grows at 48 ℃ and initial pH of 2.0, strain reaches the stable growth stage after being cultured for 3 d, and the cell density achieves the highest value (8.2×107 /mL). At 45 and 51 ℃, the cell density decreases to a large extent. At 39 and 54 ℃, the strain growth is inhibited, and the cell density is lower than 107/mL. The time reaching stable growth stage is delayed to 6 d and 4 d, respectively. Futhermore, 60 and 35 ℃ are lethal to strain growth. Therefore, the optimum temperature for strain ZW-1 growth is 48 ℃.
3.2.2 Optimal initial pH value for growth
When strain ZW-1 grows at initial pH of 1.9 and growth temperature of 48 ℃, the strain growth achieves an optimal state, and the cell density is 8.7×107 /mL. At the pH value higher than 3.0, its growth is inhibited and even lethal, which indicates that strain ZW-1 cannot grow in high pH value. At the pH value lower than 1.4, slight growth is observed, and cell density is (0.6-2.8)× 107 /mL, which shows that strain ZW-1 is a acidophilic bacteria. It is reported that, lots of acid are produced during the oxidation of sulphur and the hydrolysis of ferric iron to ferric species such as Fe(OH)2+ and Fe(OH)2+ at the last stage of chalcopyrite bioleaching, which results in low pH value (even lower than 1.3)[2, 8]. Therefore, only the acidophilic microorganisms could survive in this circumstance. The acidophilic characteristic of strain ZW-1 is potential for its bioleaching of chalcopyrite.
3.2.3 Effect of organic compounds on strain growth
The effect of organic compounds on strain ZW-1 growth is shown in Fig.2. It is shown that the strain growth is inhibited with the addition of glucose, and with the increase of glucose, cell density decreases quickly and bacteria tend to be dead. However, the addition of yeast extract powder or peptone could favor its growth. When 0.4 g/L yeast extract powder or peptone was added into the medium, cell density would increase to 8.3×107 /mL or 1.0×108 /mL. Therefore, addition of some nitric organic compounds such as yeast extract and peptone could promote the strain growth.

Fig.2 Effect of organic compounds on cell growth of strain ZW-1
3.2.4 Growth curve of strain ZW-1
The strain ZW-1 was cultured under the optimum growth condition mentioned above. The experiment was performed at 48 ℃ and pH 1.9. 5% of the seed culture growing in stable stage was inoculated to medium with addition of 0.4 g/L peptone and 30 g/L FeSO4·7H2O.
The growth curve of strain ZW-1 is shown in Fig.3. The results show that in the 24 h after inoculation, strain stays in a lag phase, and the cell density is lower than 7.1×106 /mL. From 24th hour and on, the strain grows into a exponential stage till the 64th hour, where the cell density reaches the maximum (1.1×108 /mL). When the growth continues to 80 h, the cell density begins to decrease and it is 7×107 /mL at the 104th hour.
The cell density of strain ZW-1 could reach the maximum in 72 h (1.1×108 /mL), when it grows under the optimum condition. Compared with the un-optimal experiment (48 ℃, initional pH 2.0, additon of 0.4 g/L yeast extract powder) which reveals a cell density of 8.3×107 /mL, the optimal experiment has more effective bacterial growth.
3.2.5 Utilization of substrates
Strain ZW-1 can grow autotrophically by using ferrous iron or elemental sulfur as sole energy source. The strain is also able to grow heterotrophically by using peptone and yeast extract powder, but not glucose. In addition, strain can grow by oxidizing pyrite and chalcopyrite.

Fig.3 Growth curve of strain ZW-1 at pH 1.9 and 48 ℃
3.3 Phylogenetic analysis of 16S rRNA
The 16S rRNA gene sequence of strain ZW-1 (approximately 1 461 bp) was submitted to GenBank with the accession number EF101930. Phylogenetic relationships based on 16S rRNA gene sequences are described in Fig.4. Strain ZW-1 is closely relative to Sulfobacillus acidophilus with 99.1% sequence similarity.
It is reported that[9-10], Sulfobacillus spp. is classified as facultative autotrophic bacterium. It can grow autotrophically by using ferrous iron and heterotrophically by using yeast extract powder. Furthermore, it can use elemental sulfur as energy source in the presence of yeast extract. However, only a few species in this genus could use elemental sulfur as sole energy source, such as Sulfobacilus acidophilus. Sulfobacilus acidophilus is a gram-negative, moderately thermophilic acidophilic bacterium, and it always oxidizes Fe2+ as energy source.

Fig.4 Phylogenetic tree derived from 16S rRNA sequence of strain ZW-1
Considering the morphological, biochemical, physiological characterizations and the analysis based on 16S rRNA gene sequence, strain ZW-1 is identified to be most closely related to Sulfobacilus acidophilus.
3.4 Bioleaching experiment
3.4.1 Oxidation of Fe2+
As shown in Fig.5, all Fe2+ could be oxidized to Fe3+ in 72 h by strain ZW-1. The oxidation mainly occurs at 54-60 h, when the Fe2+ oxidation rate achieves 0.295 g/(L·h). It is reported that Sulfobacillus spp. has high capability of oxidizing Fe2+[11-12]. DENG and RUAN [13] investigated the effects of several factors on oxidizing Fe2+ by Sulfobacillus thermosulfidooxidans (DSM 9293). The results showed that when the initial pH value is 2.0 and inoculation amount is 10%, the average Fe2+ oxidation rate achieves the maximum (0.360 g/(L·h)). While Fe2+ oxidation rate by mesophiles was rather slow, such as Acidithiobacillus ferrooxidans, and its maximum oxidization rate was only 0.105 g/(L·h) [14].

Fig.5 Fe2+ oxidation curve of strain ZW-1 at pH 2.0 and 48 ℃
3.4.2 Tolerance to Fe3+ and Cu2+
Since high concentrations of Fe3+ and Cu2+ would inhibit the microorganism growth in bioleaching process, the investigation on the tolerance of strain ZW-1 to these metal ions is important. The experimental results show that with the increase of Fe3+ and Cu2+ concentrations, the growth of strain ZW-1 is inhibited gradually, even though slight biomass is observed. When the concentrations of Fe3+ and Cu2+ increase to 25 and 35 g/L, respectively, strain growth disappears and the seed culture is dead after being inoculated for 24 h. Recently, the reports about the tolerance of Sulfobacillus spp. to Fe3+ and Cu2+ are rather few. While compared with other moderately thermophilic microorganisms[15-17], such as Leptospirillum ferriphilum, strain ZW-1 has higher tolerance to Fe3+ and Cu2+.
3.4.3 Bioleaching of chalcopyrite
Results of bioleaching of chalcopyrite with strain ZW-1 are shown in Fig.6. In the whole process, copper extraction continuously increases. In the first 11 d, copper extraction rate increases quickly and the copper concentration reaches 1.42 g/L. From the 12th day to the 20th day, copper extraction rate slows down. At the end of bioleaching, copper concentration is 1.64 g/L. The concentration of Fe2+ also increases continuously at the first 10 d, and then stays stable from the 10th day to the 14th day, decreases from the 14th day to the 20th day, finally reaches 431 mg/L.

Fig.6 Variations of Cu2+ and Fe2+ concentrations during bioleaching of chalcopyrite with 12 g/L pulp density at pH 2.0 and 48 ℃
The first 10 d is the main stage of bioleaching of chalcopyrite with strain ZW-1, as Cu2+ and Fe2+ extractions increase remarkably. After that, copper extraction becomes slow. In addition, the concentration of Fe2+ decreases after the 14th day. It is reported that during the bioleaching of chalcopyrite, the precipitation of jarosite and sulphur compounds (by forming a layer on mineral surface) may hinder the chalcopyrite dissolution [18]. The decrease of Fe2+ concentration is due to the fact that the formation of Fe3+ deposit would lead to reduction of Fe3+ concentration in solution, which leads to an accelerated Fe2+ oxidation[18].
In this experiment, the concentration of soluble lead in the bio-pulp is very low, which is due to the fact that the leached lead from galena mostly combines with SO42- to form PbSO4 deposition. In previous studies, it is found that PbSO4 covered on the mineral surface is a key factor for passivation of chalcopyrite, which decreases the dissolution of chalcopyrite.
Bioleaching of chalcopyrite mainly includes two reactions:
CuFeS2+O2+4H+→Cu2++Fe2++2S0+2H2O (1)
4Fe2++4H++O2→4Fe3++2H2O (2)
At the beginning of bioleaching, sulfuric acid is added into reactor to keep pH value around 2.0 for acid consumption. After 6 d, since acid is produced during the oxidation of sulphur and the hydrolysis of ferric iron to ferric species such as Fe(OH)2+and Fe(OH)2+[2], the pH decreases to a final value of 1.32.
In the bioleaching of chalcopyrite with strain ZW-1, copper extraction increases quickly at the first stage. However, since the formation of passivation layer on the mineral surface, mineral dissolution is hindered at the last stage. In a word, strain ZW-1 could extract copper of 1.64 g/L in 20 d, and the copper extraction is 46.4%, which indicates that as a pure culture, strain ZW-1 has attained good performance in bioleaching of chalcopyrite.
WITNE and PHILLIPS[19] used Sulfobacillus accidophilus (YTF1) to bioleach low-grade chalcopyrite mineral (Concentration of chalcopyrite was lower than 20%), and the copper extraction achieved 70%-80% in only 4 d. PAIVI et al[9] introduced a mixed culture including Sulfobacillus acidophilus and Sulfobacillus yellowstonensis to extract chalcopyrite concentrate. Copper extraction reached more than 90% in three months. This indicated that Sulfobacillus acidophilus cooperating with other moderate thermophiles could obtain high copper extraction in the bioleaching of chalcopyrite ores.
4 Conclusions
1) A moderately thermophilic acidophilic iron- oxidizing bacterium ZW-1 is isolated form Dexing mine, Jiangxi Province, China. According to the investigation of the morphological, biochemical and physiological characteristics and the analysis based on 16S rRNA gene sequence, this strain is identified to be most closely related to Sulfobacilus acidophilus.
2) Strain ZW-1 has strong Fe2+ oxidation ability, and the maximum of Fe2+ oxidation rate achieves 0.295 g/(L·h). It can tolerate high concentrations of Fe3+ and Cu2+, which are up to 25 g/L and 35 g/L, respectively. As a pure culture, bioleaching of chalcopyrite with strain ZW-1 could attain good performance with 46.4% of copper extraction. It can be worthy to focus further attention on the cooperative bioleaching with Sulfobacillus acidophilus and other moderate thermophiles for extracting copper from chalcopyrite.
References
[1] RIVERA-SANTLLAN R E, BALLESTER PEREZ A, BLAZQUEZ A. Bioleaching of a copper sulphide flotation concentrate using mesophilic and thermophilic microorganism [J]. Biohydrometallurgy and the Environment toward the Mining of the 21st Century, 1999(parA): 149-158.
[2] SANDSTORM A, PETERSSON S. Bioleaching of a complex sulphide ore with moderate thermophilic and extreme thermophilic microorganism [J]. Hydrometallurgy, 1997, 46: 181-190.
[3] DEW D W. The application of bioleaching for recovery of Cu, Ni and Co from concentrates [C]// Inter Hydrometallurgy Conference-Leaching Science and Technology: Current Status and Future Directions. Perth, Australia, 1999: 2.
[4] GOMEZ E, BALLESTER A, GONZALEZ F, BLAZQUEZ M L. Leaching capacity of a new extremely thermophilic microorganism, Sulfolobus rivotincti [J]. Hydrometallurgy, 1999, 52: 349-366.
[5] GOMEZ E, BALLESTER A, BLAZQUEZ M L, GONZALEZ F. Silver-catalyzed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms [J]. Hydrometallurgy, 1999, 51: 17-46.
[6] DENG Jing-shi, RUAN Ren-man, WEN Jian-kang, SUN Xue-nan. Present situation and prospects in bioleaching of sulphides by moderate thermophiles [J]. Multipurpose Utilization of Mineral Resources, 2002, 8(2): 33-38.
[7] LAN Xin-hua. The new development of gold and basic metal bioleaching [J]. Rare Metals, 2002, 7(5): 28-32.
[8] AHONEN L, TUOVINEN O H. Bacterial leaching of complex sulfide ore samples in bench-scale column reactors [J], Hydrometallurgy, 1995, 37: 1-21.
[9] PAIVI H. M, KINNUNEN J, PUHAKKA A. Characterization of iron- and sulphide mineral-oxidizing moderately thermophilic acidophilic bacteria from an Indonesian auto-heating copper mine waste heap and a deep South African gold mine [J]. Journal of Industrial Microbiology & Biotechnology, 2004, 31: 409-414.
[10] NORRIS P R, CLARK D A, OWEN J P, WATERHOUSE S. Characteristics of sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral-sulphide-oxidizing bacteria [J]. Microbiology, 1996, 142: 775-783.
[11] MELAMUD V S, PIVOVAROVA T A, TOUROVAT P, KOLGANOVAT V, OSIPOVG A, LYSENKOA M, KONDRAT'EVAT F, KARAVAIKOG I. Sulfobacillus sibiricus sp. nov., a new moderately thermophilic bacterium [J]. Microbiology, 2003, 72(5): 605-612.
[12] SAMPSON M I, PHILLIPS C V, BLAKERC. Influence of the attachment of acidophilic bacteria during the oxidation of mineral sulfides [J]. Minerals Engineering, 2000, 13(4): 373-389.
[13] DENG Jing-shi, RUAN Ren-man. The effects of factors on sulfobacillus themosulfidooxidans growth and its Fe2+ oxidization [J]. Multipurpose Utilization of Mineral Resources. 2002, 9(3): 38-41.
[14] NEMATI M, HARRIWON S T L. A comparative study of thermophile and mesophile bioleaching of ferrous iron [J]. Minerals Engineering, 2000, 13(1): 19-24.
[15] KEELING S E, PALMER M L, CARACATSANIS F C. Leaching of chalcopyrite and sphalerite using bacteria enriched from a spent chalcocite heap [J]. Minerals Engineering,2005, 18(13/14): 1289- 1296.
[16] LI Ya-qin, HE Zheng-guo. Study on Fe2+ oxidation by moderately thermoacidophilic iron-oxidizing bacteria[J]. Nonferrous Metals, 2003, 55(1): 37-39.
[17] KINNUNEN PAIVI H M, PUHAKKA JAAKKO A. High-rate iron oxidation at below pH 1 and at elevated iron and copper concentrations by a Leptospirillum ferriphilum dominated biofilm [J]. Process Biochemistry, 2005, 40(11): 3536-3541.
[18] STOTT M B, WATLING H R, FRANZMANN P D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching [J]. Minerals Engineering, 2000, 13(10/11): 1117-1127.
[19] WITNE J Y, PHILLIPS C V. Bioleaching of Ok Tedi copper concentrate in oxygen- and carbon dioxide-enriched air [J]. Minerals Engineering, 2001, 14(1): 25-48.
Foundation item: Project(2004CB619204) supported by the National Basic Research Program of China; Project(50621063) supported by the National Natural Science Foundation of China; Project(DYXM-115-02-2-07) supported by China Ocean Mineral Resources Research and Development Association
Corresponding author: ZHOU Hong-bo; Tel: +86-731-8877216; E-mail: zhouhb@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60256-3
(Edited by YANG Bing)