固溶处理对7075铝合金第二相演变和力学性能的影响
来源期刊:中国有色金属学报(英文版)2017年第10期
论文作者:邹秀亮 闫洪 陈小会
文章页码:2146 - 2155
关键词:固溶处理;显微组织演变;第二相;力学性能;7075铝合金
Key words:solution treatment; microstructure evolution; second phases; mechanical properties; 7075 Al alloy
摘 要:利用扫描电镜(SEM)、能谱(EDS)、差示扫描量热仪(DSC)、硬度测试及拉伸测试研究固溶处理工艺对铸造7075铝合金第二相演变和力学性能的影响。结果表明:随着固溶温度和时间的增加,Mg(Zn,Cu,Al)2相逐渐溶解到基体内,Al7Cu2Fe相由于熔点高,其形貌和尺寸基本没有发生变化。继续升高温度和延长时间,则开始出现粗大黑色Mg2Si颗粒。合金经460 °C固溶5 h处理,其显微硬度、抗拉强度和伸长率相比基体合金分别提高55.1%、40.91%和109.1%。这是因为此时共晶相Mg(Zn,Cu,Al)2基本完全溶解,且基本没有粗大黑色Mg2Si颗粒出现。
Abstract: The effects of solution treatment on the evolution of the second phases and mechanical properties of 7075 Al alloy were studied with scanning electron microscopy (SEM), energy dispersive X-ray spectrometry (EDS), differential scanning calorimetry (DSC), hardness and tensile tests. The results show that Mg(Zn,Cu,Al)2 phases gradually dissolve into the matrix, yet the size and morphology of Al7Cu2Fe phase exhibit no change with the increase of the solution treatment temperature and time due to its high melting point. When the solution treatment temperature and time continue to increase, the formation of coarse black Mg2Si particles occurs. Compared to the as-cast alloy, the microhardness, tensile strength, and elongation of the sample under solution heat treatment at 460 °C for 5 h are increased by 55.1%, 40.9% and 109.1%, respectively. This is because the eutectic Mg(Zn,Cu,Al)2 phases almost completely dissolve and basically no coarse black Mg2Si particles are formed.
Trans. Nonferrous Met. Soc. China 27(2017) 2146-2155
Xiu-liang ZOU1,2, Hong YAN1,2, Xiao-hui CHEN1,2
1. School of Mechanical and Electrical Engineering, Nanchang University, Nanchang 330031, China;
2. Key Laboratory of Light Alloy Preparation and Processing in Nanchang City, Nanchang 330031, China
Received 4 May 2016; accepted 31 August 2016
Abstract: The effects of solution treatment on the evolution of the second phases and mechanical properties of 7075 Al alloy were studied with scanning electron microscopy (SEM), energy dispersive X-ray spectrometry (EDS), differential scanning calorimetry (DSC), hardness and tensile tests. The results show that Mg(Zn,Cu,Al)2 phases gradually dissolve into the matrix, yet the size and morphology of Al7Cu2Fe phase exhibit no change with the increase of the solution treatment temperature and time due to its high melting point. When the solution treatment temperature and time continue to increase, the formation of coarse black Mg2Si particles occurs. Compared to the as-cast alloy, the microhardness, tensile strength, and elongation of the sample under solution heat treatment at 460 °C for 5 h are increased by 55.1%, 40.9% and 109.1%, respectively. This is because the eutectic Mg(Zn,Cu,Al)2 phases almost completely dissolve and basically no coarse black Mg2Si particles are formed.
Key words: solution treatment; microstructure evolution; second phases; mechanical properties; 7075 Al alloy
1 Introduction
Due to their high strength and low density, 7075 Al alloys are widely used in aerospace, military, and automobile industries [1-4]. However, the residual coarse and insoluble secondary phases will decrease the strength and hardness of the alloy. The solution heat treatment process influences the content, morphology and distribution of the coarse secondary phases, thus leading to the change in the mechanical properties of the alloy.
7075 Al alloy is a heat treatable Al-Zn-Mg-Cu alloy. Indeed, due to aging precipitates, T6 heat treatment is a common process to enhance the mechanical properties of the alloy. Nevertheless, the distribution and size of precipitates mainly depend on an optimal solution heat treatment process. Accordingly, choosing the proper solution treatment condition is very important to obtain the improved mechanical properties of the alloy. Additionally, the evolution of the microstructure has a direct effect on the mechanical properties of the alloy during the solution heat treatment.
In recent years, the microstructures in the as-cast and solution-treated Al-Zn-Mg-Cu alloys have been studied. In particular, it has been reported that several intermetallic phases such as η(MgZn2), T(Al2Mg3Zn3), S(Al2CuMg), θ(Al2Cu), Al7Cu2Fe, Al13Fe4 and Mg2Si can occur during solidification [5,6]. Most of the investigations were focused on the evolution of these intermetallic phases during solution heat treatment [7-9]. For instance, PENG et al [10] studied the evolution of the second phase particles during the heating-up process of the Al-Zn-Mg-Cu alloy and showed that the evolution of the second phase particles consisted of three stages: the precipitation of MgZn2 at the low temperature range, the dissolution of MgZn2 and coarsening of Al2CuMg at the medium temperature range, and the dissolution of Al2CuMg at the high temperature range. XU et al [11] observed that MgZn2 particles can be completely dissolved after holding at 475 °C for only 5 min, whereas the dissolution of Al2CuMg particles was relatively difficult in 7150 Al alloy. Meanwhile, in Ref. [12], it is mentioned that Al2CuMg phase gradually disappeared and finally dissolved into the matrix completely at 490 °C, and Al7Cu2Fe phase exhibited no change in 7050 Al alloy during the solution heat treatment. This may be resulted from the difference of chemical composition of alloy or the heat treatment condition. Although the microstructure in the as-cast condition and the evolution of the eutectic phases during the solution heat treatment have been studied in the Al-Zn-Mg-Cu alloys, only a small amount of works have researched the microstructure evolution of 7075 Al alloy during solution heat treatment. Moreover, the mechanism of the transformation from the eutectic structure to the coarse phases and the relationship between the evolution of the microstructure and mechanical properties of the alloy are entirely not clear.
The aim of this work is to evaluate the effect of solution treatment on the evolution of the second phases of 7075 Al alloy, thus analyzing the mechanical properties of the corresponding alloy. Besides, the mechanism of the transformation from the eutectic structure to Mg2Si phases was also analyzed. In addition, aging treatment was carried out in order to further show the rationality of the solution treatment process. This work has a guiding significance for further increasing the strength of the alloy.
2 Experimental
The material used in this work was prepared through a casting method in the laboratory. The chemical composition of the alloy is listed in Table 1. The solidus and liquidus temperatures of 7075 Al alloy are 477 °C and 635 °C, respectively [13].
Table 1 Chemical composition of 7075 Al alloy (mass fraction, %)

The rod-like as-cast samples were 10 mm in diameter and 110 mm in length. The solution heat treatment process was carried out in a box-type resistance furnace with temperature control. The as-cast ingots were cut into small samples with the diameter of 10 mm and the height of 10 mm. To study the influence of the solution heat treatment temperature on the microstructure of the alloy, some samples were solution-treated at temperatures of 450, 460, 470 and 480 °C for 5 h. To study the influence of solution heat treatment time on the microstructure of the alloy, other samples were solution-treated at the optimal temperature (460 °C) for 4, 5, 6 and 7 h. After the solution heat treatment, all of the samples were subsequently quenched in water at 25 °C. Following the solution heat treatment, some samples were aged at 120 °C for 24 h in order to further prove that the second phases were dissolved into the matrix after solution heat treatment.
Hardness measurements conducted on a HV-1000 microhardness tester were used to evaluate the Vickers hardness of the samples. To eliminate the possibility of the error, at least seven random hardness readings were taken for each sample. According to China’s National Standard GB/T 228-2002, the tensile samples were machined into tensile bars with a gauge length of 45 mm and a diameter of 9 mm. The tensile test was carried out at room temperature on an UTM5105 tensile test machine at a constant strain rate of 0.001 s-1. To ensure the reliability of the measured data, at least five specimens were tested for each condition.
The melting temperatures of the eutectic phases in the as-cast and solution-treated samples were determined by a Perkin-Elmer differential scanning calorimetry (DSC) at a heating rate of 10 °C/min. A Tescan-Vega3 scanning electron microscopy (SEM) equipped with energy dispersive X-ray spectrometry (EDS) was used to analyze the microstructure in the as-cast and solution-treated samples. To ensure the reliability of the EDS phase identification, EDS measurements were conducted at least nine times for each phase in three different microstructural fields. For scanning electron examinations, the samples were ground, polished, and then etched with 0.5% (volume fraction) hydrofluoric acid water for up to 5 s before rinsing with distilled water.
3 Results and discussion
3.1 Microstructures of as-cast alloy
The as-cast microstructure of 7075 Al alloy reveals a non-dendritic structure, as shown in Fig. 1(a). The microstructure consists mainly of the matrix α(Al) and the grain-boundary eutectic phases, as shown in Fig. 1(b). Actually, the eutectic phases are continuously distributed along the grain boundaries. The result of the EDS analysis shown in Fig. 1(c) indicates that the lamellar eutectic phase on the grain boundary is Mg(Zn,Cu,Al)2. Some previous investigations [14,15] revealed that if the Zn/Mg ratio of Al-Zn-Mg-Cu alloy was more than 2.2, the dominant second phase in the solidification eutectic should be the MgZn2 phase. In this work, the Zn/Mg ratio of the alloy is about 2.5. Furthermore, the EDS analysis result reveals that the stoichiometry of the bright lamellar structure is 67.20% Al, 7.63% Zn, 10.04% Mg, and 4.69% Cu (molar fraction), as indicated in Fig. 1(c). This is because some Al and Cu atoms have dissolved into the MgZn2 phase to form the Mg(Zn,Cu,Al)2 phase, which has the similar crystallographic lattice constant as the MgZn2 phase. Additionally, the EDS result presented in Fig. 1(d) indicates that the stoichiometry of elongated white phase is 77.64% Al, 4% Zn, 1.54% Mg, 8.44% Cu and 4.18% Fe (molar fraction), which is close to the stoichiometry of the Al7Cu2Fe phase. Accordingly, the elongated white phase forming during the solidification is Al7Cu2Fe, which is consistent with the finding of FAN et al [16]. Thus, the microstructure of the as-cast 7075 Al alloy consists mainly of α(Al) and the grain-boundary eutectic phases Mg(Zn,Cu,Al)2 and Al7Cu2Fe.
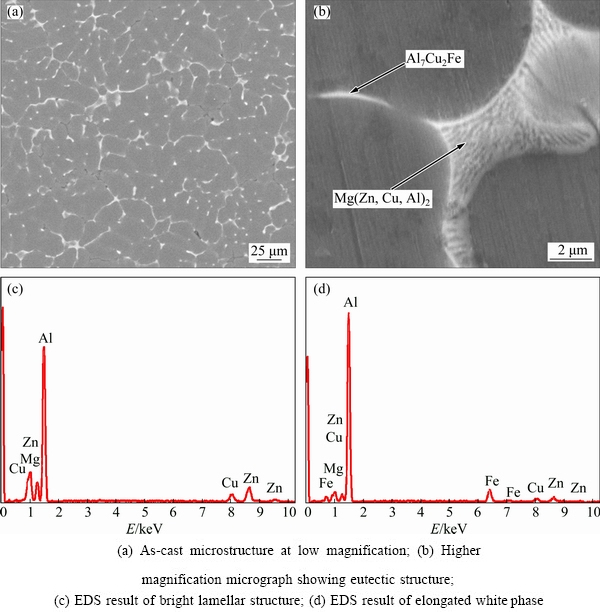
Fig. 1 Microstructure and EDS spectra of as-cast 7075 Al alloy
3.2 Effect of solution heat treatment on evolution of second phases
The evolutions of the morphology of the grain and secondary phases greatly depend on the solution treatment temperature and time. The second phases at the grain boundary evolve into isolated phases due to the dissolution of part of the second phases into α(Al) matrix after solution treatment at 450 °C for 5 h, as shown in Fig. 2(a). Meanwhile, it is evident from Fig. 2(b) that a large number of secondary phases dissolve into the α(Al) matrix and only a small number of insoluble phases are left after solutionizing at 460 °C for 5 h. When the solution heat treatment temperature continues to increase, coarse black particles form, as shown in Figs. 2(c) and (d). The results of the SEM observation and EDS spectrum analysis of 7075 Al alloy after solution treatment at 460 °C for 5 h are shown in Fig. 3. These analyses reveal that the lamellar Mg(Zn,Cu,Al)2 phase almost completely disappears and only the insoluble Al7Cu2Fe phase is left. The size and morphology of Al7Cu2Fe phase exhibit no change during the solution heat treatment due to its high melting point. The line scan analysis of the 7075 alloy in the as-cast condition and after solutionizing at 460 °C for 5 h underscores the variation of chemical composition, as shown in Fig. 4. The chemical composition in the grain boundary and intragranular region shown in Fig. 4 is listed in Table 2. The results reveal that the as-cast microstructure contains serious solute segregation in the grain boundary (points a and c), while it is poor solute in the intragranular region (point b). The solute segregation of 7075 Al alloy after solution treatment at 460 °C for 5 h almost completely disappears because the solute element diffuses homogeneously into the α(Al) matrix, as shown in Fig. 4(a). Furthermore, the chemical composition (90.47% Al, 5.44% Zn, 2.19% Mg, 1.90% Cu, mass fraction) at point d after solution heat treatment is close to the chemical composition of as-cast 7075 Al alloy, which suggests that a large number of solute elements have dissolved into the α(Al) matrix.
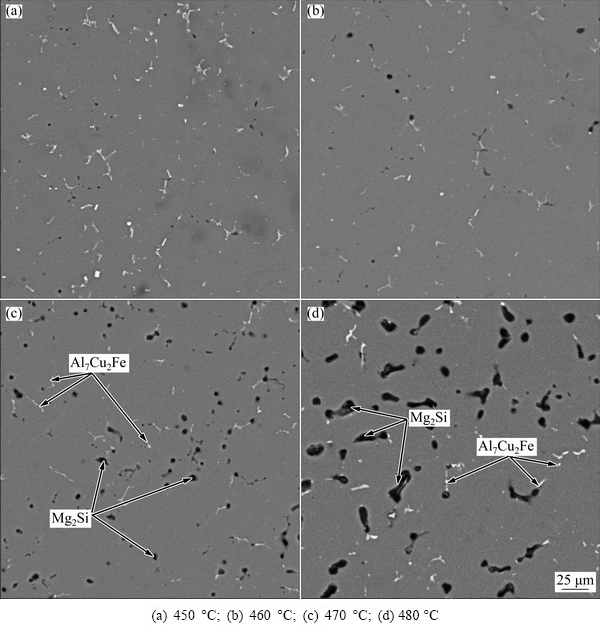
Fig. 2 Microstructures of 7075 Al alloy solution-treated at different temperatures for 5 h
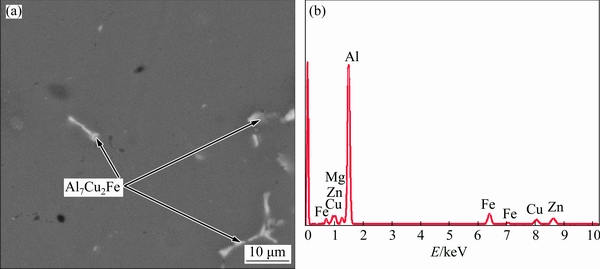
Fig. 3 SEM micrograph (a) and corresponding EDS spectrum (b) obtained from 7075 alloy after solutionizing at 460 °C for 5 h

Fig. 4 Line scan analysis results of 7075 Al alloy
Table 2 Chemical compositions at points a, b, c and d (mass fraction, %)

The results of the SEM observation and EDS spectrum of 7075 Al alloy after solution treatment at 480 °C for 5 h are shown in Fig. 5. The EDS result reveals that the stoichiometry of the coarse black particles is 16.24% Mg and 7.63% Si (molar fraction), as indicated in Fig. 5(b), which is close to the stoichiometry of Mg2Si phase. Therefore, a transformation of the eutectic phase to Mg2Si phase is observed, which is consistent with the work of MAHATHANINWONG et al [14]. In addition, the EDS result shown in Fig. 5(c) reveals that the stoichiometry of the white particles is 79.12% Al, 3.82% Zn, 1.23% Mg, 6.36% Cu and 3.32% Fe (molar fraction), which is close to the stoichiometry of Al7Cu2Fe phase. The result further proves that the size and morphology of Al7Cu2Fe phase exhibit no change during solution heat treatment due to its high melting point.
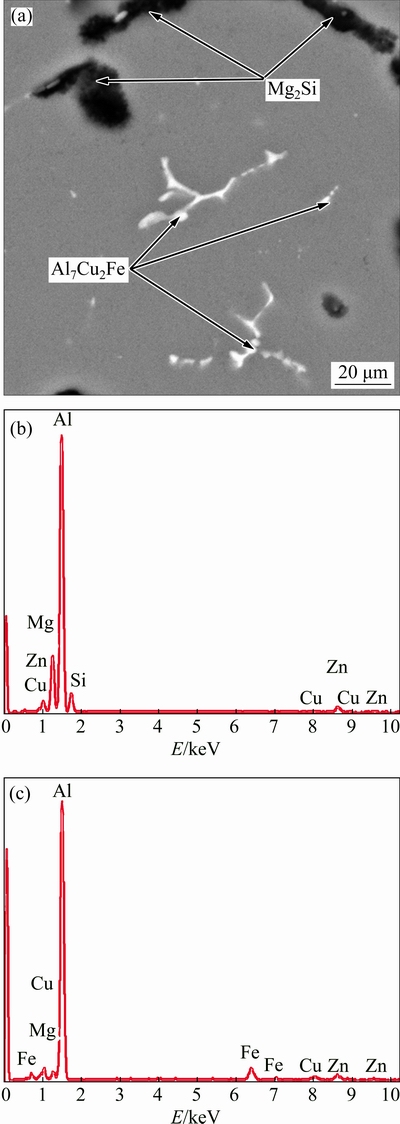
Fig. 5 SEM micrograph obtained from 7075 Al alloy after solutionizing at 480 °C for 5 h (a) and corresponding EDS spectra of coarse black phase (b) and elongated white phase (c)
As a result, only partial eutectic phases are dissolved into the α(Al) matrix under solution heat treatment at 450 °C, which results in the observed discontinuous phases. The amount of Mg(Zn,Cu,Al)2 phases dissolving into the α(Al) matrix increases with the increase of the solution heat treatment temperature. And the isolated phases in the grain boundary are clearly observed. Low melting point Mg(Zn,Cu,Al)2 phases melt when the solution heat treatment temperature continues to increase to a high enough level. The diffusion coefficient of solute elements in solids (s) is usually described by the Arrhenius equation [17]:
D=D0exp[Q/(RT)] (1)
where D0 denotes the diffusion constant; Q is the activation energy; R is the gas constant and T is the thermodynamic temperature. The diffusion parameters of some solute elements in Al(s) are presented in Table 3.
Table 3 Diffusion parameters of some solute elements in Al(s)

Accordingly, it can be calculated that the diffusion coefficients of Zn, Cu are higher than those of Mg, Si in Al(s), which causes the Zn, Cu atoms to easily dissolve into the α(Al) matrix as a solid solution. Moreover, the difference of Mg atomic radius (0.1602 nm) and Al atomic radius (0.1432 nm) is larger than that of Si atomic radius (0.1316 nm) and Al atomic radius, which easily causes lattice distortion at the surrounding of Mg atoms, thus providing the diffusion paths for Si atoms spreading to the surrounding of Mg atoms. According to Eq. (2) [22]:
△GT =△HT-T△ST (2)
where △GT is denoted as the Gibbs free energy; △H is the enthalpy change; △ST is the entropy change; T is the absolute temperature. The value of the △GT for the reaction (3) [23] is negative under this condition, which proves that the reaction can occur. In addition, Mg2Si phases easily become coarse and lead to a deterioration of the alloy properties.
2Mg(s)+Si(s)=Mg2Si(s)
△GT =-18460+3.4T<0 (450-480 °C) (3)
The microstructure of 7075 Al alloy under solution heat treatment at 460 °C for different times is shown in Fig. 6. It can be seen from Fig. 6(a) that part of the second phases are not dissolved in the sample solution treated at 460 °C for 4 h. The content of the second phases gradually decreases, while that of the black Mg2Si phase gradually increases and becomes larger with the increase of the solution time. There is nearly no change in the content of the second phases when the solution time is long enough.
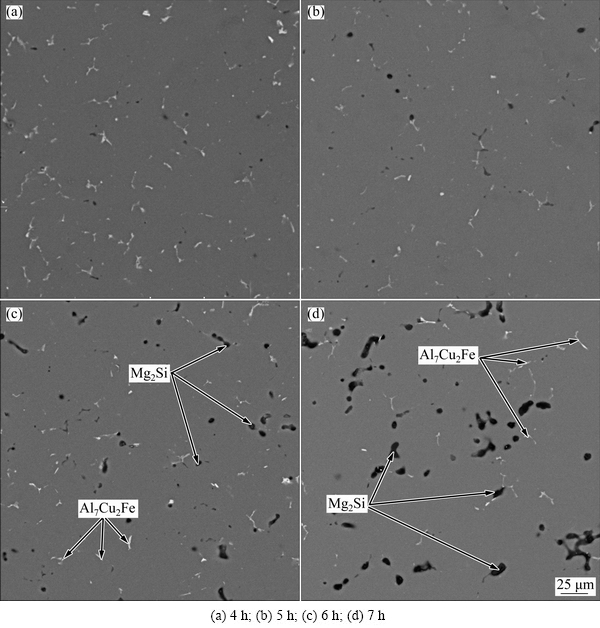
Fig. 6 Microstructures of 7075 Al alloy under solution heat treatment at 460 °C for different time
As a result, the amount of the second phase dissolving into the matrix increases with the increase of the solution time in the beginning. This is due to the fact that solute atoms need enough time to diffuse into the matrix. Additionally, when the solution time continues to increase, there is nearly no change in the content of the second phases because only insoluble phases are left. Si atoms have enough time to diffuse into the surrounding of Mg atoms and these atoms react to form the black Mg2Si phase. And the number of Mg2Si particles increases with the increase of the solution time.
The DSC plots of the 7075 Al alloy in the as-cast condition and after solutionizing at 460 °C for 5 h are shown in Fig. 7. One endothermic peak is identified at about 480 °C on the DSC plot of the as-cast sample. The endothermic peak can be ascribed to the melting of the eutectic phases (α(Al)+Mg(Zn,Cu,Al)2). After solutionizing at 460 °C for 5 h, the endothermic peak disappears, which means that almost all the Mg(Zn,Cu,Al)2 phases disappear. It is in accordance with the SEM analysis results discussed above.
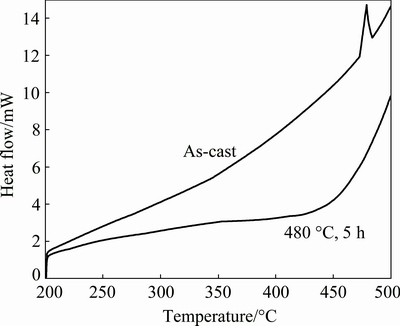
Fig. 7 DSC plots of 7075 Al alloy in as-cast condition and after solutionizing at 460 °C for 5 h
3.3 Effect of solution heat treatment on mechanical properties
The hardness values of 7075 Al alloy under different solution heat treatment temperatures for 5 h are shown in Fig. 8. It can be seen that the hardness values of 7075 Al alloy gradually increase with the increase of the solution heat treatment temperature. The hardness value of 7075 Al alloy reaches a maximum during the solution heat treatment at 460 °C, and subsequently begins to gradually decrease when the solution heat treatment temperature continues to increase. The hardness values of the alloy under solution heat treatment at 460 °C for different time shown in Fig. 9 reveal that the optimal solution heat treatment condition giving the maximum hardness value is 460 °C and 5 h. At the start, the hardness values of the alloy gradually increase with increasing solution time, while the hardness values gradually decrease when the solution time continues to increase. The tensile strength and elongation of the alloy under different heat treatment conditions are given in Table 4. It can be seen that the highest tensile strength and elongation are detected in specimens under solution heat treatment at 460 °C for 5 h during solution heat treatment, which is consistent with the variation of the hardness values of the alloy. There is a gain in the strength of the alloy aged at 120 °C for 24 h, but a loss in elongation, compared to the corresponding solution-treated alloy. As aging response depends mainly on the supersaturation degree of the matrix, aging treatment was carried out in order to further prove that the second phases dissolve into the matrix after solution heat treatment.

Fig. 8 Hardness of 7075 Al alloy solution-treated at different temperatures for 5 h
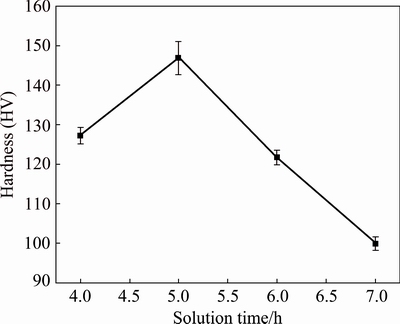
Fig. 9 Hardness of 7075 Al alloy solution-treated at 460 °C for different time
Table 4 Mechanical properties of 7075 Al alloy after different heat treatment conditions

The reasons why increasing the solution heat treatment temperature and time can increase the strength of the alloy are as follows: Firstly, the solid solubility increases with the increase of solution heat treatment temperature and time. The increase of the solid solubility leads to lattice distortion, which increases the resistance to dislocation motion. Secondly, the phase transformation driving force and nucleation rate increase, which results in the reduction of the size of the grains. In addition, the diffusion rate of the solute elements increases according to Eq. (1), which leads to an uniform distribution of the solute elements. However, the formation of coarse black Mg2Si particles occurs when the solution heat treatment temperature and time continue to increase. Such particles can reduce the strength and hardness of the alloy. This is because the coarse black Mg2Si particles would hinder the dissolution of the solute elements into the matrix, which results in the lowering of the supersaturation solid solution in the solution-treated sample. In addition, grain growth occurs with the increase of the solution heat treatment temperature and time, which has an unfavorable effect on the mechanical properties of the alloy.
The SEM micrographs of the fracture surfaces of 7075 Al alloy after different heat treatment conditions are shown in Fig. 10. It can be seen from Fig. 10(a) that the fracture surface of the as-cast 7075 Al alloy reveals distinctly ductile intergranular rupture. The sample shows evidence of low strength and strain, which is in accordance with the mechanical properties of the as-cast alloy (Table 4). This is due to the fact that precipitates at the grain boundaries during solidification lead to the reduction in the grain-boundary strength. The SEM fractograph of 7075 Al alloy under solution heat treatment at 460 °C for 5 h is shown in Fig. 10(b). It is evident that the fracture surface reveals predominantly quasi-cleavage rupture, including many tearing ridges, steps and ductile dimples. It is noted that the sample experiences a higher plastic deformation during cracking, which is consistent with the uniform microstructure and superior mechanical properties of the solution-treated sample. Figure 10(c) shows the SEM fractograph of 7075 Al alloy aged at 120 °C for 24 h after solution heat treatment at 460 °C for 5 h. It can be found that the fracture surface also reveals mostly quasi-cleavage rupture, but only includes few tearing ridges. Due to fine aging precipitations restraining dislocation motion in the tensile deformation process, the plastic property of the alloy shows evidence of reduction.
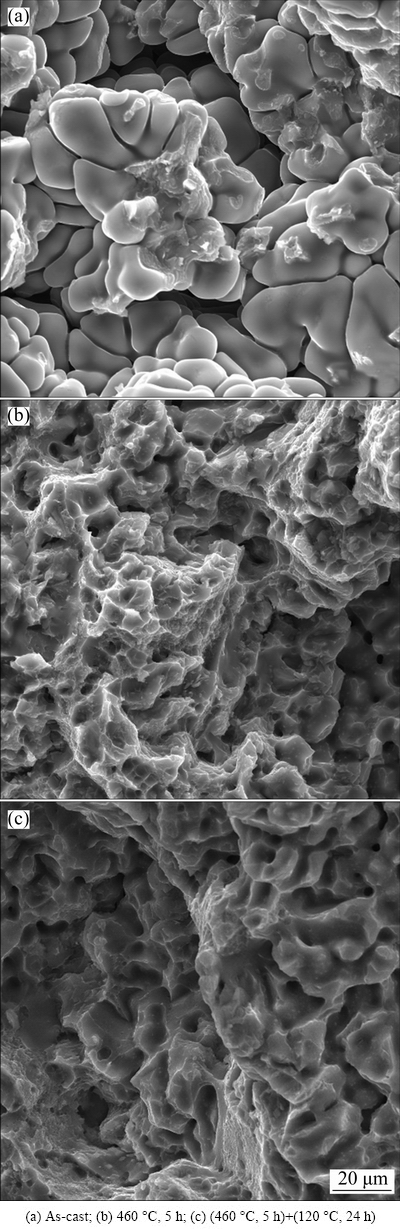
Fig. 10 SEM morphologies of fracture surfaces of 7075 Al alloy after different heat treatments
4 Conclusions
1) With the increase of solution heat treatment temperature and time, Mg(Zn,Cu,Al)2 phase gradually dissolves into the α(Al) matrix, while Al7Cu2Fe phase remains. Due to its high melting point, the size and morphology of Al7Cu2Fe exhibit nearly no change. When the solution treatment temperature and time continue to increase, the formation of coarse black Mg2Si particles occurs. This is due to the fact that the diffusion coefficients of Zn and Cu atoms are larger than those of Mg and Si atoms.
2) The optimum solution heat treatment condition for 7075 Al alloy is 460 °C and 5 h. Compared to the as-cast alloy, the microhardness (HV 146.82), tensile strength (285.22 MPa), and elongation (2.3%) of the solution-treated sample are increased by 55.1%, 40.9% and 109.1%, respectively.
3) The fracture surface of the sample solution treated at 460 °C for 5 h reveals predominantly quasi-cleavage rupture. The dissolution of secondary phases leads to the lowering of the resistance to dislocation motion during the solution heat treatment, which can improve the plasticity of the alloy. And the fracture surface of the sample aged at 120 °C for 24 h after solution treatment at 46 °C for 5 h also mostly reveals quasi-cleavage rupture. Due to the presence of fine aging precipitations, the plasticity of the alloy is reduced, which is consistent with the few tearing ridges observed in the fractography.
References
[1] CHEN Xiao-hui, YAN Hong, JIE Xiao-ping. Effects of Ti addition on microstructure and mechanical properties of 7075 alloy [J]. International Journal of Cast Metals Research, 2014, 28(3): 151-157.
[2] ZHANG Fei, SU Xue-kuan, CHEN Zi-yong, NIE Zuo-ren. Effect of welding parameters on microstructure and mechanical properties of friction stir welded joints of a super high strength Al-Zn-Mg-Cu aluminum alloy [J]. Materials and Design, 2015, 67: 483-491.
[3] CHEN Gang, CHEN Qiang, WANG Bo, DU Zhi-ming. Microstructure evolution and tensile mechanical properties of thixoformed high performance Al-Zn-Mg-Cu alloy [J]. Metals and Materials International, 2015, 21(5): 897-906.
[4] SUN Yi-shan, JIANG Fu-lin, ZHANG Hui, SU Jian, YUAN Wu-hua. Residual stress relief in Al-Zn-Mg-Cu alloy by a new multistage interrupted artificial aging treatment [J]. Proceedings of the National Academy of Sciences, 2016, 92(23): 281-287.
[5] CONG Fu-guan, ZHAO Gang, JIANG Feng, TIAN Ni, LI Rui-feng. Effect of homogenization treatment on microstructure and mechanical properties of DC cast 7X50 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1027-1034.
[6] ROKHLIN L L, DOBATKINA T V, BOCHVAR N R, LYSOVA E V. Investigation of phase equilibria in alloys of Al-Zn-Mg-Cu-Zr-Sc system [J]. Journal of Alloys and Compounds, 2004, 367(1-2): 10-16.
[7] LI Chun-mei, CHEN Zhi-qian, ZENG Su-min, CHENG Nan-pu, CHEN Tian-xiao. Intermetallic phase formation and evolution during homogenization and solution in Al-Zn-Mg-Cu alloys [J]. Science China Technological Sciences, 2013, 56(11): 2827-2838.
[8] LIU Tao, HE Chun-nian, LI Gen, MENG Xin, SHI Chun-sheng. Microstructural evolution in Al-Zn-Mg-Cu-Sc-Zr alloys during short-time homogenization [J]. International Journal of Minerals Metallurgy and Materials, 2015, 22(5): 516-523.
[9] LI Bo, PAN Qing-lin, CHEN Cong-ping, WU Hai-hua, YIN Zhin-min. Effects of solution treatment on microstructural and mechanical properties of Al-Zn-Mg alloy by microalloying with Sc and Zr [J]. Journal of Alloys and Compounds, 2016, 664: 553-564.
[10] PENG Guo-sheng, CHEN Kang-hua, CHEN Song-yi, FANG Hua-chan. Evolution of the second phase particles during the heating-up process of solution treatment of Al-Zn-Mg-Cu alloy [J]. Materials Science and Engineering A, 2015, 641: 237-241.
[11] XU D K, ROMETSCH P A, BIRBILIS N. Improved solution treatment for an as-rolled Al-Zn-Mg-Cu alloy. Part I. Characterisation of constituent particles and overheating [J]. Materials Science and Engineering A, 2012, 534(2): 234-243.
[12] LIU Jiao-jiao, LI Hong-ying, LI De-wang, WU Yue. Application of novel physical picture based on artificial neural networks to predict microstructure evolution of Al-Zn-Mg-Cu alloy during solid solution process [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(3): 944-953.
[13] BOLOURI A, SHAHMIRI M, KANG C G. Study on the effects of the compression ratio and mushy zone heating on the thixotropic microstructure of AA 7075 aluminum alloy via SIMA process [J]. Journal of Alloys and Compounds, 2011, 509(2): 402-408.
[14] MAHATHANINWONG N, PLOOKPHOL T, WANNASIN J, WISUTMETHANGOON S. T6 heat treatment of rheocasting 7075 Al alloy [J]. Materials Science and Engineering A, 2012, 532: 91-99.
[15] DENG Yun-lai, WAN Li, WU Li-hui, ZHANG Yun-ya, ZHANG Xin-ming. Microstructural evolution of Al-Zn-Mg-Cu alloy during homogenization [J]. Journal of Materials Science, 2011, 46(4): 875-881.
[16] FAN Xi-gang, JIANG Da-ming, MENG Qing-chang, ZHONG Li. The microstructural evolution of an Al-Zn-Mg-Cu alloy during homogenization [J]. Materials Letters, 2006, 60(12): 1475-1479.
[17] DU Y, CHANG Y A, HUANG B, GONG W, JIN Z. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation [J]. Materials Science and Engineering A, 2003, 363(1): 140-151.
[18] HILLIARD J E, AVERBACH B L, COHEN M. Self and interdiffusion in aluminum-zinc alloys [J]. Acta Metallurgica, 1959, 7(2): 86-92.
[19] ROTHMAN S J, PETERSON N L, NOWICKI L J, ROBINSON L C. Tracer diffusion of magnesium in aluminum single crystals [J]. Physica Status Solidi (b), 1974, 63(1): 29-33.
[20] CERESARA S. A step annealing procedure for the determination of diffusion coefficients in metals by the resistometric method-application to the diffusion of Cu in Al [J]. Physica Status Solidi (b), 1968, 27(2): 517-520.
[21] DU Yong, CHANG Y A, HUANG Bai-yun, GONG Wei-ping, JIN Zhan-peng, XU Hong-hui, YUAN Zhao-hui, LIU Yong, HE Yue-hui, XIE F Y. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation [J]. Materials Science and Engineering A, 2003, 363:140-151.
[22] CHEN Xiao-hui, YAN Hong. Fabrication of nanosized Al2O3 reinforced aluminum matrix composites by subtype multifrequency ultrasonic vibration [J]. Journal of Materials Research, 2015, 30(14): 2197-2209.
[23] ZHANG Song-li, ZHAO Yu-tao, CEHN Gang. In situ (Mg2Si+MgO)/Mg composites fabricated from AZ91-Al2(SiO3)3 with assistance of high-energy ultrasonic field [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(11): 2096-2099.
邹秀亮1,2,闫 洪1,2,陈小会1,2
1. 南昌大学 机电工程学院,南昌 330031;
2. 南昌市轻合金材料制备与加工重点实验室,南昌 330031
摘 要:利用扫描电镜(SEM)、能谱(EDS)、差示扫描量热仪(DSC)、硬度测试及拉伸测试研究固溶处理工艺对铸造7075铝合金第二相演变和力学性能的影响。结果表明:随着固溶温度和时间的增加,Mg(Zn,Cu,Al)2相逐渐溶解到基体内,Al7Cu2Fe相由于熔点高,其形貌和尺寸基本没有发生变化。继续升高温度和延长时间,则开始出现粗大黑色Mg2Si颗粒。合金经460 °C固溶5 h处理,其显微硬度、抗拉强度和伸长率相比基体合金分别提高55.1%、40.91%和109.1%。这是因为此时共晶相Mg(Zn,Cu,Al)2基本完全溶解,且基本没有粗大黑色Mg2Si颗粒出现。
关键词:固溶处理;显微组织演变;第二相;力学性能;7075铝合金
(Edited by Xiang-qun LI)
Foundation item: Project (51364035) supported by the National Natural Science Foundation of China; Project (CX2015055) supported by the Innovation Special Funds of Nanchang University for Graduate Student, China
Corresponding author: Hong YAN; Tel: +86-791-83968633; E-mail: yanhong_wh@163.com
DOI: 10.1016/S1003-6326(17)60240-1

