Bacillus sp. G1产生物表面活性剂对含铅港口底泥的修复
来源期刊:中国有色金属学报(英文版)2017年第6期
论文作者:郭一明 刘云国 李华 郑爱兵 谭小飞 张明明
文章页码:1385 - 1393
关键词:铅;底泥;表面活性剂;修复
Key words:plumbum; sediment; surfactant; remediation
摘 要:研究了一株新分离的Bacillus sp. G1所产生物表面活性剂对含铅港口底泥的修复,及在不同固水比、pH、离子强度等条件下对铅的去除效果。结果显示:可交换态铅淋滤后最大吸附去除量可达76.8 mg/g;Langmuir等温吸附模型可以更好地反映该生物表面活性剂对Pb的吸附特性;傅立叶变换红外吸收光谱仪分析显示C=O及 —CH3可能是有效功能基团;扫描电镜观察发现沉积物样品表面结构在活性剂处理前后由粗糙变光滑,反映了铅离子与表面活性剂间的络合更稳定。研究表明,菌株Bacillus sp. G1所产表面活性剂对铅有较好的去除效果,为含铅港口底泥修复提供了新的思路。
Abstract: The remediation of Pb-contaminated port sediment by biosurfactant from a new isolated Bacillus sp. G1 was studied. The Pb removal efficiencies were investigated under multi-levels of water-solid ratio, pH and ionic strength. Result showed that exchangeable speciation of Pb could be removed by maximum removal capacity of 76.8 mg/g after leaching. The Langmuir isotherm reflected the adsorption process best to fit the experimental adsorption equilibrium data. Fourier transform infrared spectra (FTIR) indicated that C=O and —CH3 may be the functional groups. Scanning electron microscopy (SEM) analysis showed that the surface of the port sediment became much smoother after adsorption interaction, which reflected that the complexation between Pb ions and biosurfactant was more stable. The results indicated that the biosurfactant of Bacillus sp. G1 could remove Pb effectively from the Pb-contaminated port sediment (PCPS) and suggested a novel method for PCPS remediation.
Trans. Nonferrous Met. Soc. China 27(2017) 1385-1393
Yi-ming GUO1, Yun-guo LIU2,3, Hua LI1, Ai-bing ZHENG1, Xiao-fei TAN2,3, Ming-ming ZHANG2,3
1. School of Economics and Management, Shanghai Maritime University, Shanghai 201306, China;
2. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China;
3. Key Laboratory of Environmental Biology and Pollution Control, Ministry of Education, Hunan University, Changsha 410082, China
Received 31 March 2016; accepted 12 April 2017
Abstract: The remediation of Pb-contaminated port sediment by biosurfactant from a new isolated Bacillus sp. G1 was studied. The Pb removal efficiencies were investigated under multi-levels of water-solid ratio, pH and ionic strength. Result showed that exchangeable speciation of Pb could be removed by maximum removal capacity of 76.8 mg/g after leaching. The Langmuir isotherm reflected the adsorption process best to fit the experimental adsorption equilibrium data. Fourier transform infrared spectra (FTIR) indicated that C=O and —CH3 may be the functional groups. Scanning electron microscopy (SEM) analysis showed that the surface of the port sediment became much smoother after adsorption interaction, which reflected that the complexation between Pb ions and biosurfactant was more stable. The results indicated that the biosurfactant of Bacillus sp. G1 could remove Pb effectively from the Pb-contaminated port sediment (PCPS) and suggested a novel method for PCPS remediation.
Key words: plumbum; sediment; surfactant; remediation
1 Introduction
Plumbum (Pb) was identified by the World Health Organization (WHO) as one of the toxic substances, which threatens the ecosystem and human’s central nervous, cardiovascular, gastrointestinal, reproductive, renal and immune systems [1]. Currently, the world population is still exposed to a dangerous level of environmental plumbum [1,2]. Not only the mining or smelting, but also the transporting of nonferrous metal ores will result in Pb entering into the biosphere [3]. Previous researches have proved that, Pb in sediment was mostly from anthropogenic sources such as coal combustion, automobile emission, dust of lead-zinc ores and river catchment. Apart from those inputs, the neighboring large-scale ore ports and aerosols also resulted in serious Pb pollution in port sediment [4-6]. Many ports in the world are facing the Pb-contamination problem, such as Western Harbor of Alexandria [7], East London and Port Elizabeth harbours [8], main harbours of the Galician Rias [9], Xiawan Port in China [10]. Being one of the greatest nonferrous metal producers, China also suffers heavy Pb pollution from the nonferrous metal ore-port or transporting. Previous studies have demonstrated that Pb-contaminated sediment distributed widely in Chinese coastal areas, such as Bohai Bay [4], urban Victoria and Tolo Harbour of Hong Kong [11] and Southern East China Sea.
Pb-contaminated port sediment (PCPS) will do great harm to human health along with the food chain [12], and will affect the regional environmental quality or bring food security issues [13,14]. Thus, the removal of Pb ions from PCPS is quite important for protecting public health and environment. Biosurfactant is a kind of surface-active biological macromolecule substance that was produced by microorganisms (including glycolipids, lipopeptides, lipoprotein, phospholipids, and neutral lipid derivatives). Compared with the chemical surfactants, biosurfactant has advantages in reducing the oil water interfacial tension, emulsifying, foaming and froth breaking [15].
Biosurfactant is also biodegradable and harmless to the environment [16]. By carrying the hydrophobic and hydrophilic groups simultaneously, biosurfactant is much easier to combine with the hydrophobic organic compounds, so that it can effectively adsorb Pb ions in PCPS [17-20]. The feasibility of using biosurfactants for some kinds of heavy metal removal from sediments was already proved [21]. For instance, surfactin from Bacillus subtilis, rhamnolipids from Pseudomonas aeruginosa and sophorolipid from Torulopsis bombicola were proven effectively to remove copper or zinc in sediments [22]. The potential of biosurfactant from marine bacterium for the remediation of heavy metals also turned out to be strong. Previous research has already demonstrated the properties of biosurfactant that chelate heavy metals and form insoluble precipitate [23]. Thus, biosurfactant may find tremendous application in treatment of heavy metal-containing wastewater [23]. Port sediments usually contain higher clay and organic matters, which are rather easy to adsorb Pb, therefore, those methods applying in soil Pb remediation may not be proper for PCPS, and biosurfactant may be a strategy for the treatment of PCPS [3,24].
In this study, a biosurfactant producing bacterial strain was isolated successfully from the leachate of an ore stacking yard, and this strain was intended to belong to Bacillus sublitis comprehensively according to its physiological biochemical properties and 16S rDNA sequence analysis. The feasibility study of using the biosurfactant from this Bacillus sublitis to remove Pb in PCPS was first investigated. Some experimental factors including the water-solid ratio (w/s), initial pH and ionic strength were further discussed. Three kinds of classical isotherm models were used to simulate the biosorption characterization. The overarching objective of this work is to provide a novel Bacillus sublitis to promote the biosurfactant method for the remediation of Pb in PCPS.
2 Experimental
2.1 Materials
2.1.1 Medium
To fulfill the needs of this research, 5 types of media were prepared as follows.
1) Enrichment medium. Glucose (5 g), peptone (5 g), K2HPO4 (2 g), distilled water (1000 mL), pH=7-7.2.
2) Solid plate culture medium. Beef extract (3 g), peptone (10 g), NaCl (5 g), agar (15-20 g), distilled water (1000 mL), pH=7-7.2.
3) Seed medium. Beef extract (3 g), peptone (10 g), NaCl (5 g), distilled water (1000 mL), pH=7-7.2.
4) Fermentation medium. Olive oil (20 g), NaNO3 (8 g), K2HPO4 (3 g), KH2PO4 (3 g), NaCl (5 g), trace element solution (4 mL), distilled water (1000 mL), pH=7.
5) Trace element solution. CaCl2 (2 mg/L), FeCl3·6H2O (50 mg/L), CuSO4 (0.5 mg/L), MnCl2·4H2O (0.5 mg/L), ZnSO4·7H2O (10 mg/L).
2.1.2 Collection of sediment
The sediment samples were collected from the siltation of a coastal harbor which is near the port mineral yard and received the leachate of Pb-rich ores. The pH value of this PCPS is 7.8. The sediment samples were dried slowly in the shade, pulverized by a stick, and then sieved through 200 mesh screens. Particle size distribution of this PCPS was 17% sand, 73% silt and 15% clay. The content of organic matter in the sediment is 5.6%, and content of Pb is 0.08526 mg/g.
2.2 Screening of strains
Functional bacteria strain was screened following the steps below.
1) Two bottles of 100 mL enrichment medium were autoclaved at 121 °C for 15 min. Leachate water from an ore dock was inoculated according to the inoculation amount of 5% at 37 °C, and cultured for 1 d with shaking. Afterwards, enrichment cultivation was replicated 3 times.
2) 0.2 mL of the enriched culture was injected into the solid plate culture medium, and the mixture was cultured at 37 °C until bacterial colonies thrived. To separate the individual bacterial strain, repeated plate streaking approach was taken. The single strain was successfully derived using the inclined plane method and then stored at 4 °C in refrigerator for further use.
3) Inoculate the first or second ring of the selected strain on the inclined plane into the seed medium. Time to reach stationary phase, subsequently, the bacterial strain was inoculated into the fermentation medium in the amount of 2%. After 3 d fermentation at 37 °C, 160 r/min shaking cycles, bacteria with lager colony diameter and oil spreading were selected to test the surface tension after filtration and centrifugation separation.
4) Bacteria strain which showed the maximum oil spreading and surface tension reduction was picked for physiological biochemical properties testing. The 16S rDNA sequence analysis method was taken for further identification before the picked bacteria strain was taken as the surfactant producing bacteria for later researches.
2.3 Extraction of biosurfactant
The fermentation liquid was filtered after 3 d culture, and then the filtrate was centrifuged at the rotation speed of 9000 r/min for 30 min. After the centrifugation, the supernatant was filtered once again and its pH value was adjusted to 2, then it was extracted by equivalent volume of mixture solution (volume ratio of chloroform to methanol is 3:1) 3 times. The organic solvent was collected after extraction and was prepared for evaporation on the rotary evaporator until 10 mL left. After natural volatilization, the residual solid product was used to adopt for 8 h CH2Cl2 extraction at 50 °C. For the next step, organic phase was evaporated for a second time on the rotary evaporator to 20 mL still at 50 °C, and then was poured out until 20 mL remaining naturally volatilized till the crude surfactant products were finally obtained [25,26].
2.4 Influential factors of Pb removal
In each test with different solution concentrations, 1 g of PCPS was added into 20 mL biosurfactant solutions at 25 °C. Then, the mixtures were put in an incubator shaker with a shaking speed of 160 r/min for 48 h. After the samples were taken out, they were centrifuged at 3000 r/min for 30 min. The supernatant was removed, and the concentration of Pb in the aqueous phase was measured. The pH was adjusted with hydrochloric acid and sodium hydroxide, and the above process was repeated when changing the other influence factors including pH, inorganic salt, leaching time and reaction time, correspondingly. By repeating the above determining process, water phase concentration of Pb was also obtained accordingly.
3 Results and discussion
3.1 Identification of strain
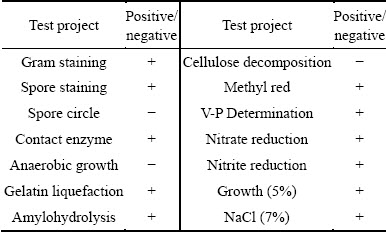
The observation was done under the microscope, and in the eyepiece vision, the strain cells were arranged in a straight shape, either alone or in pairs. The bacterial colony was roughly round with irregular edge and dry surface, and turned green after being inoculated into beef extract peptone culture medium for 1 d. Gram staining and spore staining of the bacterial colony were both positive, and the spores were middle or terminal and oval shaped. The physiological biochemical characteristics of this strain are shown in Table 1.
Table 1 Physiological-biochemical characteristics of Bacillus sp. G1
The screened strain was then identified by determination of 16S rDNA gene sequences, and was found to belong to the genus Bacillus. The highest homology obtained was 100% of similarity to Bacillus subtilis. The 16S rDNA sequence was submitted to the GenBank and given the accession number AB018486. The phylogenetic tree based on a multiple sequence alignment of the 16S rDNA sequence is presented in Fig. 1. This strain was named as Bacillus sp. G1.
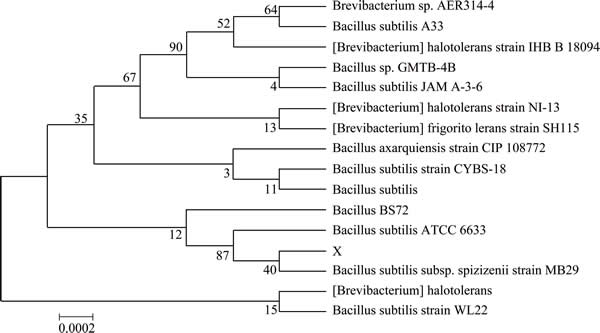
Fig. 1 Phylogenetic tree established by neighbor-joining method based on 16S rDNA sequence of Bacillus sp. G1 and similar sequence obtained from NCBI
3.2 Effects of water-solid ratio on Pb removal
Figure 2 shows the effects of different water-solid ratios on the biosurfactant’s PCPS-Pb removal (after vibrating for 48 h, pH=3).
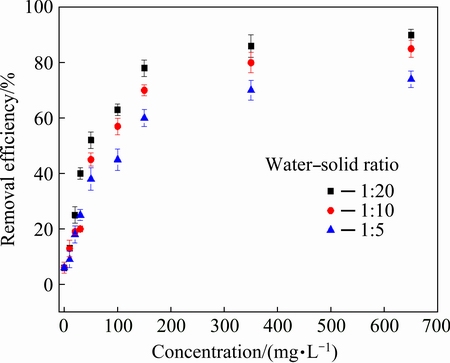
Fig. 2 Effect of water-solid ratio on Pb removal efficiency
Figure 2 reveals that the removal efficiency of Pb varied with the water-solid ratio. More specifically, the Pb removal efficiency enhanced with the increasing amount of surfactant when dealing with the equal mass of PCPS, i.e. the order of the Pb removal efficiencies under various water-solid ratio was: 1:20 > 1:10 > 1:5. The possible reason is that with the dosage and liquid phase volumetric growth of biosurfactant, the accommodation spaces of biosurfactant on the Pb ions enlarged accordingly. Additionally, the enlarged area of contact might also result in full connection between the Pb ions and biosurfactants.
3.3 Effects of pH on Pb removal
Different Pb removals were investigated under initial surfactant concentration of 320 mg/L, vibration for 48 h, pH values of 2-10. Figure 3 shows the influence of pH of biosurfactant solution on Pb removal.
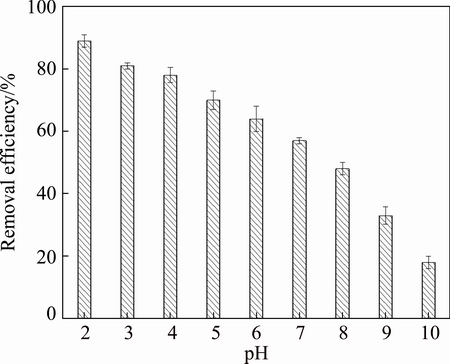
Fig. 3 Effect of pH of biosurfactant solution on Pb removal
Quite different removal efficiencies under different pH levels can be observed in Fig. 3. The removal capacity gradually decreased with the increase of pH value, and the highest Pb removal occurred at the pH value of 2.0. When the biosurfactant solution was acidic, the structure of microaggregates in the sediments would be destroyed, and the Pb ions could not be effectively bound to the sediment. Then, Pb ions on the surface of the sediment got increasingly, which could make it more liable to react with the biosurfactant micelle so that the Pb ions were effectively precipitated out. In alkaline condition, Pb ions were prone to precipitate on the surface of sediment, which greatly reduced the amount of Pb ions in the biosurfactant. Meanwhile, under strong alkaline conditions, the higher pH would make the micelle of the biosurfactant structure smaller [27], which would make it more ineffective to combine with Pb ions.
3.4 Effects of background ionic concentration on Pb removal
Two ordinary inorganic salt NaCl and MgCl2 were used to investigate the effects of inorganic salt ions on Pb removal efficiency when the initial biosurfactant concentration was 320 mg/L, pH value of 3. Figure 4 shows the effect of the inorganic salts on Pb removal.
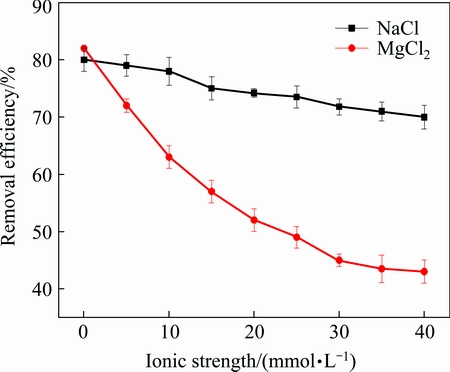
Fig. 4 Effect of inorganic salt on Pb removal
As shown in Fig. 4, the Pb removal was less affected by NaCl than by MgCl2. By observing the ionic strength of MgCl2 on the Pb removal in Fig. 4, it could be seen that the Pb removal capacity decreased rapidly at first before the ionic strength reached 30 mmol/L, but after the ionic strength exceed 30 mmol/L, the decrease of Pb removal capacity tended to be gentle. One possible reason for relatively weak removal capacity under NaCl ionic strength background, might be due to the fact that Na+ carried less charge than Mg2+ thus occupied less bonding points on the biosurfactant surface, which limited the removal of Pb ions. For Mg2+, because it carried more charge, it could form precipitation with the biosurfactant [28], which weakened the effects of the biosurfactant on the removal capacity of Pb.
3.5 Removal of various forms of Pb by biosurfactant at different pH values
There are usually diverse forms of Pb in the PCPS. According to BCR 3-state extraction method proposed by European Community Standard Measurement and Testing Organization, the morphologies of Pb in PCPS were analyzed properly. The results showed that Pb in PCPS had basically exchangeable form, Fe-Mn oxide binding form, carbonate bound form and residue form. Different forms of Pb tend to have disparate bonding forces and might result in different removal capacities. Figure 5 shows the removal of various forms of Pb by biosurfactant at different pH values.
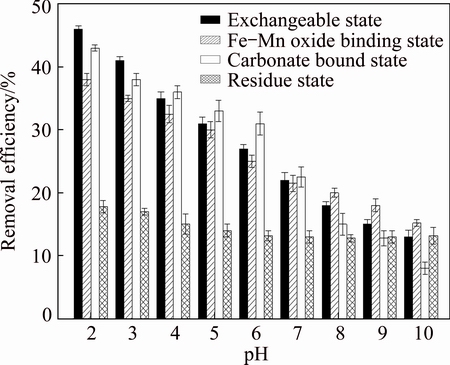
Fig. 5 Various forms of Pb removal at different pH values
Figure 5 shows that for all forms of Pb, the removal capacity decreased with increasing pH. The best removal capacity for each form of Pb was obtained at pH 2.0, the removal capacity was in the following order: exchangeable state (45.89%) > carbonate bound state (43.28%) > Fe-Mn oxide binding state (38.78%) > residue state (18.26%).
When the biosurfactant solution was in acidic environment, the Pb-salt solution could be hydrolyzed and would form a low solubility metal hydroxide, which meant that under strong acidic condition, the acidic hydration hydrogen ions and the acidic Pb ions on the alkaline sediment surface would raise a strong adsorption competition. And this adsorption competition would further promote the desorption of Pb ions, and resulted in more Pb ions resolved from the sediments. However, when the biosurfactant solution was in alkaline conditions, more Pb precipitation would form and less binding site would retain on the metal surface. Thus, due to the weak strength of adhesion force between biosurfactant and Pb ions, the dissolving of Pb ions from the PCPS precipitation would then be stopped.
3.6 Sorption isotherm analysis
The kinetic and thermodynamic characteristics of biosurfactant (from Bacillus sp. G1) adsorption were systematically studied for Pb ions. The results of Langmuir, Freundlich and Dubinin-Raduskevich isotherms are shown in Fig. 6.
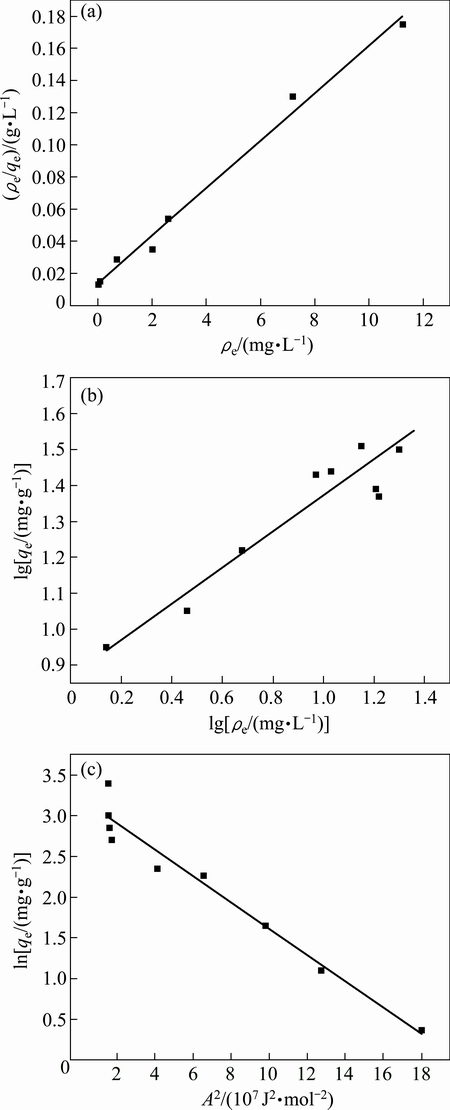
Fig. 6 Langmuir adsorption isotherm (a), Freundlich adsorption isotherm (b) and Dubinin-Raduskevich adsorption isotherm (c)
The linear form of the Langmuir model could be expressed as [29,30]
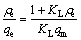 (1)
(1)
The linear form of the Freundlich model could be expressed as
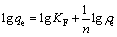 (2)
(2)
The linear form of the Dubinin-Raduskevich model could be expressed as
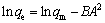 (3)
(3)
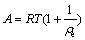 (4)
(4)
where ρe (mg/L) and qe (mg/g) represent the equilibrium concentration and the equilibrium adsorption capacity of biosurfactant for Pb2+, respectively; qm (mg/g) is the maximum adsorption capacity for Pb2+; KL (L/mg) is the Langmuir constant related to the adsorption heat that released from the affinity of adsorbent and the adsorbate at the binding sites; KF (mg/g) is the Freundlich adsorption coefficient that is related to the adsorption capacity; n is an index of isotherm nonlinearity that is related to the adsorption intensity; B is the adsorption isothermal constant of Dubinin-Raduskevich, which represents adsorption free energy of per unit (mol2/J2); A (J/mol) is the Polanyi-adsorption energy which is defined as the energy for adsorbing a mole of molecules from infinity towards the adsorption sites; R (J/(mol·K) stands for the gas constant and T (K) stands for adsorption temperature.
A statistic of the model parameters is listed accordingly in Table 2.
As shown in Table 2, the values of the correlation coefficients indicated a better fitting to Langmuir adsorption isotherm with the experimental data compared with the adsorption isotherm Freundlich and Dubinin-Raduskevich, and the maximum adsorption capacity of the Langmuir model was 76.8 mg/g. The Langmuir model assumes that a monomolecular layer was formed when adsorption takes place without any interaction between the adsorbed molecules [31]. The results of this study indicated that Pb ions were adsorbed by specific sites of biosurfactant (from Bacillus sp. G1) and formed monolayer. The adsorption process might involve multiple mechanisms, such as ion-exchange, electrostatic attraction and surface complexation [32].
3.7 Combination mechanism analysis
In order to illustrate the combination mechanism between Pb ion and biosurfactant, the Fourier transform infrared (FT-IR) spectra of biosurfactant before and after adsorption of Pb were analyzed. The results are presented in Fig. 7.
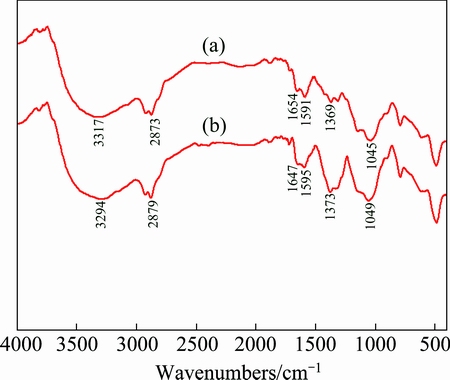
Fig. 7 FT-IR spectra of biosurfactant before (a) and after (b) Pb adsorption
As illustrated in Fig. 7, FT-IR spectra of the biosurfactant indicated that carboxyl and hydroxyl groups were present in abundance. These groups might function as proton donors, and hence the deprotonated hydroxyl and carboxyl groups may be involved in coordination with Pb2+ [33]. The intense band at 3317 cm-1 was ascribed to the O—H stretching vibrations of hydrogen-bonded hydroxyl groups. The peak at 1654 cm-1 was ascribed to the C=O stretching vibration. The intense band at 1369 cm-1 was assigned to the bending vibration of —CH3 groups. After adsorption of Pb, the band at 3317 cm-1 shifted to 3294 cm-1, which supported the complexation between Pb2+ and hydroxyl function groups [33,34]. Moreover, the band at 1045 cm-1 was shifted to 1049 cm-1, indicating coordination of Pb2+ with carboxylate groups [33]. The intensities of the peaks at 1654 cm-1 and 1369 cm-1 shifted to 1647 cm-1 and 1373 cm-1 after the biosorption process, respectively, indicating that C=O and —CH3 functional groups also played important roles in Pb2+ biosorption [35].
3.8 SEM analysis
Figure 8 shows that the significant changes occurred on the surface of PCPS before and after treatment with the biosurfactant.
Table 2 Adsorption equilibrium constants obtained from Langmuir, Freundlich and Dubinin-Raduskevich isotherms

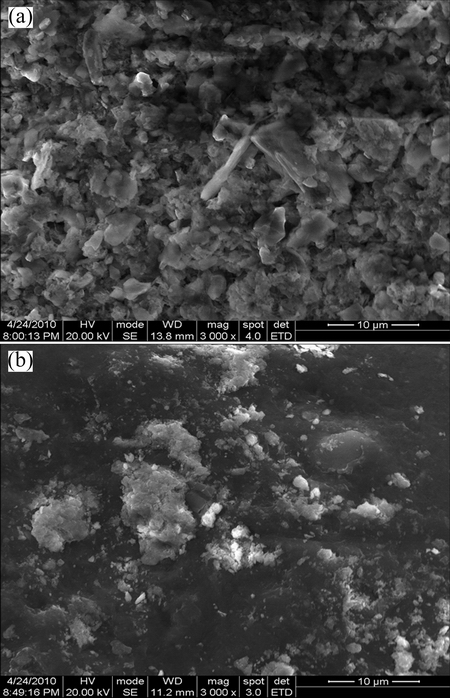
Fig. 8 SEM images of sediment surface before (a) and after (b) treatment
The change of the PCPS surface structure was well reflected in Fig. 8. As shown in Fig. 8(a), before the treatment of biosurfactant, the surface of PCPS was rough and uneven, indicating that Pb ions were bounded by soil particles. However, it became smoother and had less metal binding sites after the treatment (Fig. 8(b)). This result demonstrated that a large amount of Pb was removed after the treatment of biosurfactant solution. Possible reasons may explain these changes as fellows. Firstly, the dissociate Pb ions were chelated into the complexation with the macromolecule of biosurfactant. Secondly, the adsorption sites on the surface of the PCPS particles were much weaker than the competitive adsorption from the biosurfactant, and the complexation that formed with Pb ions and biosurfactant was more stable which promoted the migration of Pb ions from the PCPS.
4 Conclusions
1) A biosurfactant producing bacterial strain was isolated successfully from the leachate of an ore stacking yard, and this strain was named as Bacillus sp. G1 after the physiological-biochemical properties analysis and 16S rDNA sequence identification.
2) Biosurfactant of Bacillus sp. G1 has a notable effective removal on the Pb ions in PCPS, and the higher the water-solid ratio was, the better the removal capacity would be. The pH value of the purification system largely affects the Pb removal capacity. The highest Pb removal efficiency occurred when the pH was 2.0, but with the increase of pH value from 2.0 to 10.0, Pb removal decreased gradually. Pb removal efficiency was less affected by NaCl, but relatively more affected by MgCl2 under different inorganic salt concentration backgrounds.
3) Among diverse forms of Pb in the PCPS, the exchangeable states achieved the highest Pb removal efficiency (45.89%) at the pH value of 2.0. Under acidic conditions, the biosurfactant also had a certain removal capacity for Pb in the Fe-Mn oxide bound or the carbonate bound states, whereas the residual state was the smallest.
4) According to the SEM images, it could be noted that the surface of sediment was rough and rugged before leaching, but the surface of PCPS became smoother after biosurfactant leaching. The results indicated that the biosurfactant of Bacillus sp. G1 could remove Pb effectively from the Pb-contaminated port sediment (PCPS), and suggested a novel method for PCPS remediation.
References
[1] OMELCHENKO A. Environmental lead contamination as eco-terrorism and a threat to ecosystems and public health [J]. Nato Security Through Science, 2011, 112: 83-99.
[2] SYUKOR A R A, SULAIMAN S, SIDDIQUE M N I, ZULARISAM A W, SAID M I M. Integration of phytogreen for heavy metal removal from wastewater [J]. Journal of Cleaner Production, 2016, 112: 3124-3131.
[3] MULLIGAN C N, YONG R N, GIBBS B F. Heavy metal removal from sediments by biosurfactants [J]. Journal of Hazardous Materials, 2001, 85: 111.
[4] HU Ning-jing, HUANG Peng, ZHANG Hui, ZHU Ai-mei, LIU Ji-hua, ZHANG Jun, HE Lian-hua. Anthropogenic Pb input into Bohai Bay, China: Evidence from stable Pb isotopic compositions in sediments [J]. Continental Shelf Research, 2015, 109: 188-197.
[5] LIANG Jing, MAO Jian-su. Risk assessment of lead emissions from anthropogenic cycle [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 248-255.
[6] WU Chao, PENG Xiao-lan, WU Guo-min. Wetting agent investigation for controlling dust of lead-zinc ores [J]. Transactions of Nonferrous Metals Society of China, 2007,17: 159-167.
[7] SAAD M A, BELTAGY A I, MAHMOUD W M. Total dissolved and particulate lead in the western Harbor of Alexandria, a Mediterranean basin under stress [J]. Marine Pollution Bulletin, 2003,47: 52-58.
[8] FATOKI O S, MATHABATHA S. An assessment of heavy metal pollution in the east London and Port Elizabeth harbours [J]. Water SA, 2001, 27: 233-240.
[9] PREGO R P, FERRO P, TRUJILLO C. Lead and zinc contamination of surface sediments in the main harbours of the Galician Rias [J]. Journal of Iberian Geology, 2008, 34: 243-252.
[10] ZHU Hui-na, YUAN Xing-zhong, ZENG Guang-ming, JIANG Min, LIANG Jie, ZHANG Chang, YIN Juan, HUANG Hua-jun, LIU Zhi-feng, JIANG Hong-wei. Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1470-1477.
[11] MCIWEM S N, HUI Y H, LAM P K S. Pollution in the coastal waters of Hong Kong: Case studies of the urban Victoria and Tolo Harbours [J]. Water and Environment Journal, 2011, 25: 387-399.
[12] LE H T, NGO H T T. Cd, Pb, and Cu in water and sediments and their bioaccumulation in freshwater fish of some lakes in Hanoi, Vietnam [J]. Toxicological and Environmental Chemistry, 2013, 95: 1328-1337.
[13] MARTIN R, DOWLING K. Trace metal contamination of mineral spring water in an historical mining area in regional Victoria, Australia [J]. Journal of Asian Earth Sciences, 2013,77: 262-267.
[14] RIEDER S R, TIPPING E, ZIMMERMANN S, GRAF-PANNATIER E, WALDNER P, MEILI M, FREY B. Dynamic modelling of the long term behaviour of cadmium, lead and mercury in Swiss forest soils using CHUM-AM [J]. Sci Total Environ C, 2014, 468-469: 864-876.
[15] PERINELLI D R, CASETTARI L, CESPI M, FINI F, MAN D K W, GIORGIONI G, CANALA S, LAM J K W, BONACUCINA G, PALMIERI G F. Chemical–physical properties and cytotoxicity of N-decanoyl amino acid-based surfactants: Effect of polar heads [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 492: 38-46.
[16] ASADOLLAHI L, SALEHIZADEH H, YAN N. Investigation of Biosurfactant activity and Asphaltene biodegradation by Bacillus cereus [J]. Journal of Polymers & the Environment, 2016, 2: 119-128.
[17] CHEN Yu-cheng, XIONG Zhi-ting, DONG Shan-yan. Chemical behavior of cadmium in purple soil as affected by surfactants and EDTA [J]. Pedosphere, 2006, 16: 91-99.
[18] GEORGIOU G, LIN S C, SHARMA M M. Surface-active compounds from microorganisms [J]. Bio/technology, 1992, 10: 60-65.
[19] MAO Xu-hui, JIANG Rui, XIAO Wei, YU Jia-guo. Use of surfactants for the remediation of contaminated soils: A review [J]. Journal of Hazardous Materials, 2015, 285: 419-435.
[20] VANALAKAR S A, SURYAWANSHI M P, MALI S S, MOHOLKAR A V, KIM J Y, PATIL P S, KIM J H. Simplistic surface active agents mediated morphological tweaking of CdS thin films for photoelectrochemical solar cell performance [J]. Current Applied Physics, 2014, 14: 1669-1676.
[21] MOGHADDAM M Y, SHAFAEI S Z, NOAPARAST M, ARDEJANI F D, ABDOLLAHI H, RANJBAR M, SCHAFFIE M, MANAFI Z. Empirical model for bio-extraction of copper from low grade ore using response surface methodology [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 4126-4143.
[22] MULLIGAN C N, YONG R N, GIBBS B F. Heavy metal removal from sediments by biosurfactants [J]. Journal of Hazardous Materials, 2001, 85: 111-125.
[23] DAS P, MUKHERJEE S, SEN R. Biosurfactant of marine origin exhibiting heavy metal remediation properties [J]. Bioresource Technology, 2009, 100: 4887-4890.
[24] LIU Yun-guo, FAN Ting, ZENG Guang-ming, LI Xin, TONG Qing, YE Fei, ZHOU Ming, XU Wei-hua, HUANG Yu-e. Removal of cadmium and zinc ions from aqueous solution by living Aspergillus niger [J]. Transactions of Nonferrous Metals Society of China, 2006, 16: 681-686.
[25] CHEN Wei-chuan, JUANG Ruey-shin, WEI Yu-hong. Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms [J]. Biochemical Engineering Journal, 2015, 103: 158-169.
[26] YAN Yang, CHEN Ke-bin, LI Hao-ran, HONG Wei, HU Xiao-bin, XU Zhou. Capping effect of reducing agents and surfactants in synthesizing silver nanoplates [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 3732-3738.
[27] CHAMPION J T, GILKEY J C, LAMPARSKI H, RETTERER J, MILLER R M. Electron microscopy of Rhamnolipid (biosurfactant) morphology: Effects of pH, cadmium, and octadecane [J]. Journal of Colloid and Interface Science, 1995, 170: 569-574.
[28] YAN Yang, CHEN Ke-bin, LI Hao-ran, HONG Wei, HU Xiao-bin, XU Zhou. Capping effect of reducing agents and surfactants in synthesizing silver nanoplates [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 3732-3738.
[29] NAMASIVAYAM C, SURESHKUMAR M V. Modelling thiocyanate adsorption onto surfactant-modified coir pith, an agricultural solid ‘waste’ [J]. Process Safety and Environmental Protection, 2007, 85: 521-525.
[30] JAVADIAN H, AHMADI M, GHIASVAND M, KAHRIZI S, KATAL R. Removal of Cr(VI) by modified brown algae Sargassum bevanom from aqueous solution and industrial wastewater [J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44: 977-989.
[31] AKSU Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris [J]. Process Biochemistry, 2002, 38: 89-99.
[32] LI Ting-ting, LIU Yun-guo, PENG Qing-qing, HU Xin-jiang, LIAO Ting, WANG Hui, LU Ming. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling [J]. Chemical Engineering Journal, 2013, 214: 189-197.
[33] LU Huan-liang, ZHANG Wei-hua, YANG Yu-xi, HUANG Xiong-fei, WANG Shi-zhong, QIU Rong-liang. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar [J]. Water Research, 2012, 46: 854-862.
[34] LIU Yun-guo, LIAO Ting, HE Zhong-bing, LI Ting-ting, WANG Hui, HU Xin-jiang, GUO Yi-ming, HE Yuan. Biosorption of copper (II)from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1804-1814.
[35] SARANYA P, BHAVANI P, SWARNALATHA S, SEKARAN G. Biosequestration of chromium(III) in an aqueous solution using cationic and anionic biosurfactants produced from two different Bacillus sp. – A comparative study [J]. Rsc Advances, 2015, 98: 80596-80611.
郭一明1,刘云国2,3,李 华1,郑爱兵1,谭小飞2,3,张明明2,3
1. 上海海事大学 经济管理学院,上海 201306;
2. 湖南大学 环境科学与工程学院,长沙 410082;
3. 湖南大学 环境生物与控制教育部重点实验室,长沙 410082
摘 要:研究了一株新分离的Bacillus sp. G1所产生物表面活性剂对含铅港口底泥的修复,及在不同固水比、pH、离子强度等条件下对铅的去除效果。结果显示:可交换态铅淋滤后最大吸附去除量可达76.8 mg/g;Langmuir等温吸附模型可以更好地反映该生物表面活性剂对Pb的吸附特性;傅立叶变换红外吸收光谱仪分析显示C=O及 —CH3可能是有效功能基团;扫描电镜观察发现沉积物样品表面结构在活性剂处理前后由粗糙变光滑,反映了铅离子与表面活性剂间的络合更稳定。研究表明,菌株Bacillus sp. G1所产表面活性剂对铅有较好的去除效果,为含铅港口底泥修复提供了新的思路。
关键词:铅;底泥;表面活性剂;修复
(Edited by Xiang-qun LI)
Foundation item: Project (2016M590348) supported by China Postdoctoral Science Foundation; Projects (41301154, 41271332) supported by the National Natural Science Foundation of China
Corresponding author: Yi-ming GUO; Tel: +86-21-38282442; E-mail: ymguo@shmtu.edu.cn
DOI: 10.1016/S1003-6326(17)60159-6

