Inhibition mechanism between sodium (Na3AlF6) and sulfur on coke reactivity
来源期刊:中南大学学报(英文版)2017年第8期
论文作者:肖劲 仲奇凡 李发闯 LI Jie(李劼)
文章页码:1736 - 1744
Key words:mutual inhibition; sulfur; sodium; coke reactivity
Abstract: Inhibition mechanism between sodium (Na3AlF6) and sulfur on coke reactivity was investigated by simulating petroleum coke with low-impurity pitch coke and by impurity doping. The mechanism was discussed by scanning electron microscopy, energy-dispersive spectrometry, and X-ray powder diffraction. Results show that Na effectively inhibited S catalysis during carbon–air/CO2 reactions, and S inhibited the catalysis of Na during carbon– air reaction to a certain extent. A stable structure with a Na-to-S atomic ratio of 1.4 and a cyclic reaction system of “Na2SO3→ Na2S→ Na2CO3→ Na2SO3” were likely the keys to producing this mutual inhibition.
Cite this article as: ZHONG Qi-fan, XIAO Jin, LI Fa-chuang, LI Jie. Inhibition mechanism between sodium (Na3AlF6) and sulfur on coke reactivity [J]. Journal of Central South University, 2017, 24(8): 1736-1744. DOI: https://doi.org/ 10.1007/s11771-017-3581-y.
J. Cent. South Univ. (2017) 24: 1736-1744
DOI: https://doi.org/10.1007/s11771-017-3581-y
ZHONG Qi-fan(仲奇凡)1, XIAO Jin(肖劲)1, 2, LI Fa-chuang(李发闯)1, LI Jie(李劼)1, 2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. National Engineering Laboratory of Efficient Utilization of Refractory Nonferrous Metal Resources
(Central South University), Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract: Inhibition mechanism between sodium (Na3AlF6) and sulfur on coke reactivity was investigated by simulating petroleum coke with low-impurity pitch coke and by impurity doping. The mechanism was discussed by scanning electron microscopy, energy-dispersive spectrometry, and X-ray powder diffraction. Results show that Na effectively inhibited S catalysis during carbon–air/CO2 reactions, and S inhibited the catalysis of Na during carbon– air reaction to a certain extent. A stable structure with a Na-to-S atomic ratio of 1.4 and a cyclic reaction system of “Na2SO3→ Na2S→ Na2CO3→ Na2SO3” were likely the keys to producing this mutual inhibition.
Key words: mutual inhibition; sulfur; sodium; coke reactivity
1 Introduction
The content of impurity elements, which are the main raw materials of carbon anode, is an influential factor affecting the quality of carbon anode reactivity. Petroleum coke is a major component of the main structure of carbon anode (about 90% of anode quality) and also a main impurity element that is an anode source [1]. Impurity elements affect the carbon anode reaction essentially by affecting the petroleum coke reactivity. Impurity elements, including S, Ca, and V, exist in petroleum coke. S, the main impurity element for coke, is a strong catalytic impurity element for air and CO2 reactivity of coke. The impurity element S existing in petroleum coke is mainly in the forms of thiophene and thiophene derivatives, whereas the inorganic sulfur in sulfate or pyrite form is generally ≤10% [2]. It is pointed out that the sulfur content in petroleum coke is closely linked with the dibenzothiophene (DBT) content in crude coke [3, 4]. Therefore, most of the organic sulfur in petroleum coke probably comes from DBT. And when studying the effect of other impurity elements on petroleum coke without considering S element, as the main impurity element in coke (1%-10% in mass fraction, usually), S will influence the research results in many aspects.
During aluminum electrolysis process, a mass of cryolite (Na3AlF6) will contact with the carbon anode directly, which brings the Na with strong catalysis to coke [5, 6]. It has been proved that it is the Na bringing the catalysis, no Al or F. However, no clear explanation is available for Na catalytic reaction mechanism. MEIJER et al [7] proposed that if the source of Na is different, reactive catalysis strength would change. They suspected that the catalytic mechanism of Na to coke is different because of the different Na sources. KAPTEIJN et al [8] speculated that Na catalysis is probably by increasing the affinity of O2/CO2 to carbon matrix. However, as the main element of molten salt (Na3AlF6) in the process of aluminum electrolysis, the catalytic effect mechanism of Na element and the mutual inhibition mechanism between S element and it on carbon anode reactivity couldn’t be ignored and need to be studied deeply.
Based on choosing the method of using pitch coke instead of petroleum coke, by adding various sorts of chemical synthesis containing Na and S to low-impurity pitch coke and by combining several testing devices such as scanning electron microscopy (SEM), energy- dispersive spectrometry (EDS), and X-ray powder diffraction (XRD), we aimed to conduct a profound and systematic exploration into the mutual inhibition mechanism of sodium (Na3AlF6) on coke reactivity with sulfur existing.
2 Experimental section
2.1 Materials
In the experiment, a kind of low-impurity coal tar pitch was chosen as the raw material which was used in producing impurity-added coke samples. Table 1 shows some main impurity elements content of the pitch coke sample without any impurity adding.
Table 1Main impurity elements content of pitch coke
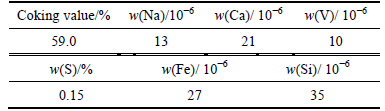
The existing form of S element in petroleum coke is mainly classed as organic S as thiophenes [2]. Meanwhile, DBT (dibenzothiophene) is deduced as organic S impurities. And this deduction was investigated firstly by ENGVOLL [9] several years ago. Engvoll is the person who firstly chose the method of using pitch coke instead of petroleum coke to study the reactivity of coke with DBT-added. Considering that Na impurities in the carbon anode mostly come from cryolite (Na3AlF6), and the elements of F and Al in Na3AlF6 can be seen as harmless and inactive during coke–air/CO2 reactions, Na3AlF6 is selected as the only source of Na for the study on the mechanism of mutual inhibition between S and Na. The Na3AlF6 used in the experiment belonged to reagent grade.
2.2 Samples preparation
The samples were prepared by a few steps. Firstly, the coal tar pitch was melted under the temperature of 200 °C. During pitch melted, the reagents which were chose as the dopants, were added in and mixed well. Then the samples were moved in to furnace reactor and calcined for one hour in advance under the temperature of 550 °C. This step was aimed to prevent the sample from melting again and then polluted. The surfaces of samples were cut and threw away, and the leaving parts were crushed into particles. Finally, the samples are coked under the temperature of 1100 °C. After coked by one hour, the samples can be obtained successfully.
2.3 Samples analysis
2.3.1 Reactivity analysis
In this work, reactivity referred to the chemical reactivity between carbon-air and carbon-CO2. Reactivity of the coke samples were tested on the condition that the equipment was under the circumstance of constant heat with 50 L/h air volume or CO2 volume as shown in Fig. 1. In the experiment, the testing samples with a mass of 5 g and a width of 1-1.4 mm were placed in reactors with temperatures of 600 °C (for air reactivity test) or 1000 °C (for CO2 reactivity test) for 1 h. The detail description of the reactivity testing analyzer and the detail testing method could be found in our previous works [10, 11]. Their reactivity can be tested by high or low change of the mass-loss rate of the coke samples after carbon-air/CO2 reaction.
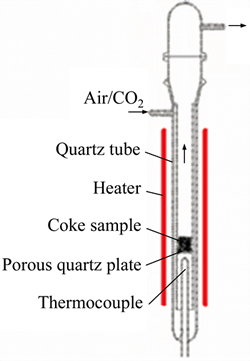
Fig. 1 Detailed description of reactivity testing analyzer
2.3.2 Other analysis
The sulfur content in coke was measured by the means of Coulomb with the aid of HDS3000 model AI Sulfur Detector. And the detector was made by Huade Electronic Ltd (Hunan Province, China).
To test SEM and EDS, the kind of FEI Quanta 200 scanning electronic microscope equipped with an accelerated voltage of 20 kV and an X-ray energy dispersive spectrometer was resorted.
To measure XRD, a D/Max 2500 diffractometer using Cu Kα line radiation (Rigaku produced) was employed.
3 Results and discussion
3.1 Effects of S-added and Na-added Na3AlF6 on coke reactivity
As shown in Table 2, by doping different amounts of Na3AlF6 and DBT into the pitch, the low-sulfur (Na3AlF6-added, named NA) samples and high-sulfur (Na3AlF6 + DBT-added, named NAS) ones were prepared. It can be seen clearly that with the quantity of DBT added was kept constant, the final S content in the pitch coke slightly increased with increased amount of added Na3AlF6 during preparation. The specific effect of “sulfur-fixed” is proved produced by Na-added. In addition, F in Na3AlF6 will volatilize during heating, which can be ignored and has no effect to coke.
Table 2 Basic parameters of samples with different Na contents
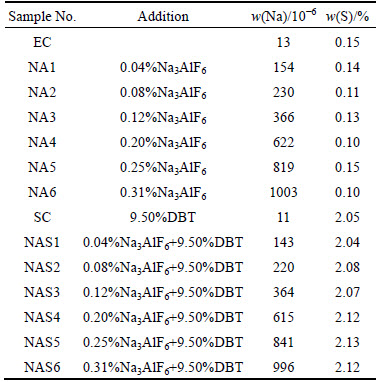
Figure 2 shows the test results of the samples after carbon-air reaction. With further increase, but it still cannot reach the air reaction residue rate of low-sulfur sample. With increased Na content (<230×10-6), the S is speculated to still have catalysis effect on coke. However, with increased Na content, the inhibition of Na to the catalysis of S on coke begins. When the Na content exceeded 230×10-6, the air reaction residue rate of the high-sulfur sample is reduced as well and is still above the air reaction residue rate of the low-sulfur sample. Nevertheless, Na clearly has an inhibition effect on the catalysis of S to coke. The result is similar to the experimental result of HUME et al [12], who had mentioned that S could weaken the sensitivity of coke to Na.
Figure 3 shows the CO2 reactivity of each sample. With increased Na content, the CO2 reaction residue of low-sulfur coke showed a slight declining trend. Thecatalysis of Na to coke is smooth. However, with increased Na content, the residual rate of high-sulfur coke after carbon–CO2 reaction initially increased and then decreased. With increased Na content from 0 to 230×10-6, the CO2 residue of high-sulfur coke sharply increased. However, when Na content exceeded 360×10-6, the CO2 residue of high-sulfur coke slightly decreased, the same as low-sulfur coke. Although the Na content increased from 0 to 1000×10-6, the CO2 reaction residue rate of high-sulfur coke is lower than that of low-sulfur coke. Clearly, Na has an inhibition catalysis effect on carbon–CO2 reaction. However, the inhibition effect of Na reaches its limit at about 300×10-6. With further increased Na content, the newly added Na resumed its reaction catalytic ability again. Nevertheless, with 2% added in the coke, S has no obvious inhibition effect on the catalytic ability of Na.
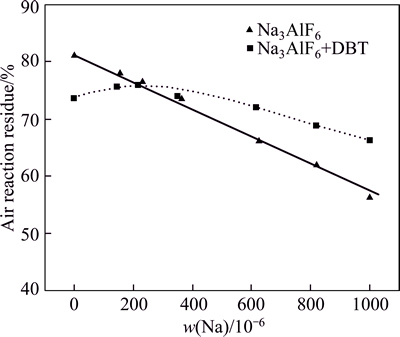
Fig. 2 Residue rate of low- and high-sulfur Na3AlF6-added cokes after carbon-air reaction
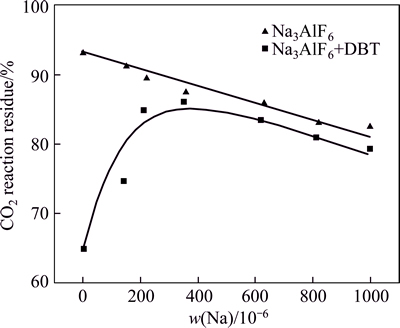
Fig. 3 Residue rate of low- and high-sulfur Na3AlF6-added cokes after carbon-CO2 reaction
According to the above experimental results, in the process of coke reaction with air and CO2, two different types of interaction behaviors maybe exist between Na and S impurities. During the carbon–air reaction, a mutual inhibition effect between S and Na occurs, whereas during the carbon–CO2 reaction, only Na has an inhibition effect on the catalysis of S unilaterally. Above all, both the two kinds of interaction behaviors are seemed to have “poor efficiency”. In both carbon–air and carbon–CO2 reactions, when the Na content in the coke exceeds 300×10-6, the residue of high-sulfur coke decreased with increased Na content. Hence, if Na content in coke is high, not all Na would be involved in the interaction behavior of Na and S, and part of Na is likely to be formed into a relatively independent free form and keep their original carbon–air/CO2 reaction catalytic ability. The experiment of Section 3.3 was carried out as followed for verifying the speculation which stated above.
3.2 Mechanism analysis of mutual inhibition between Na and S
Combining XRD, SEM, and EDS, an experiment was conducted to analyze the surface formations of the pitch coke samples, including NA6 (Na3AlF6-doped) and NAS6 (Na3AlF6+DBT-doped), by comparing them before and after air/CO2 reactivity test.
Figure 4 shows the SEM images of the two pitch coke samples before the carbon-gas reaction, whereas Table 3 details the EDS analysis results of the surface formations of the samples. As shown in Figs. 4(a) and (b), many block white bodies were detected on the surface of Na3AlF6-doped coke. The EDS analysis results show that elements C, Na, Al, F, and O can be found in these block, white body places (e.g., A1, A2, and A3), and the atomic ratio of Na-to-Al is approximately 3. Eliminating the carbon base, the block white bodies are speculated to be Na3AlF6 doped in the experiment. To verify the stated speculation, a pitch coke sample doped with 10% Na3AlF6 was analyzed by XRD, and the result is shown in Fig. 5. Only Na3AlF6 characteristic peaks existed, which means that the Na3AlF6 doped in the coke does not change during pitch coking. Therefore, the block white bodies in the sample should be Na3AlF6. As shown in Figs. 4(c) and (d), only few white needle crystal structures exist on the plane surface of NAS6 sample, and no obvious foreign matter has been found on the fracture surface. EDS analysis results show that except for C, elements Na, Al, and O can be found in the white needle crystal structures (region A4 and A5), and the atomic ratios of Na-to-Al and Na-to-O are approximately 1 and 1.5–2, respectively. No other polluting reagent is observed, thus it is speculated to be other forms, which were transformed from Na3AlF6 and named as NaAlO1.5–2.
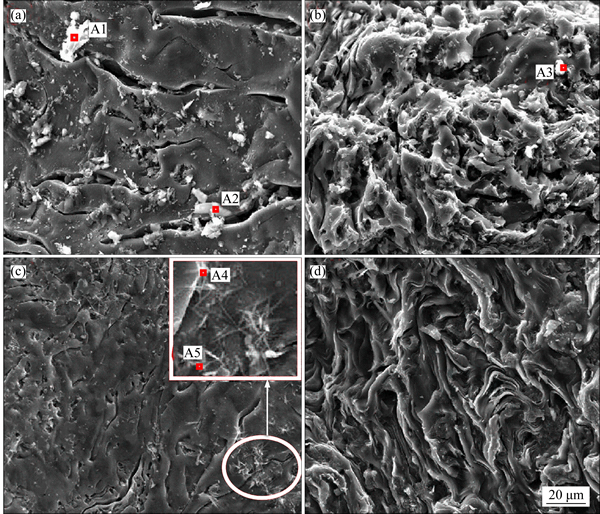
Fig 4 SEM images of plane surface of NA6 (a), fracture surface of NA6 (b), plane surface of NAS6 (c) and fracture surface of NAS6 (d)
Table 3 Element composition of area A1-A5 in Fig. 4
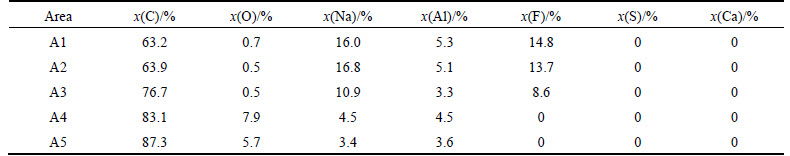
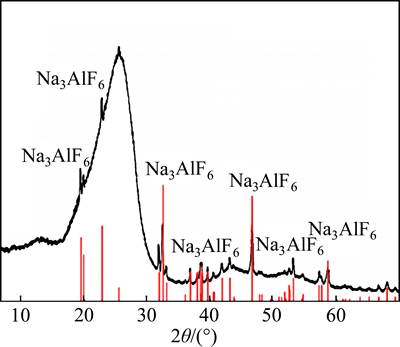
Fig 5 XRD patterns of coke sample prepared by doping 10% Na3AlF6
3.2.1 Mechanism analysis of mutual inhibition between Na and S on air reactivity
Figure 6 presents the SEM images of the two samples, namely, NA6 and NAS6, after the air reactivity tests. Table 4 shows the EDS analysis results of the foreign bodies that exist on the sample plane and fracture surfaces. As shown in Fig. 6(a) and the data of A6 and A7 in Table 4, the Na3AlF6 has been replaced by a new form of white foreign bodies after carbon–air reaction. The white foreign bodies have no F elements, but with C, O, Na, and Al, and the atomic ratio of Na-to-Al is approximately 1.6 (named Na1.6AlO). Na1.6AlO is speculated to be transformed by Na3AlF6 and has close relationship with the catalysis of Na to carbon–air reaction. As shown in Figs. 6(b) and (c), the white needle crystal structure, NaAlO1.5–2 has disappeared completely, and some white foreign bodies appeared, as shown in A11 region. The white foreign bodies are similar to Na1.6AlO in the elementary composition, but the atomic ratio of Na-to-Al is small (<0.5). The new white foreign bodies and Na1.6AlO should be the same series of matter, but in different phase compositions; to show the difference, it is named Na0.5AlO. The element S is mostly enriched in two kinds of regions: the first kind of region is the corrosion pit and holes, as shown in A9 and A10; the other kind is the corrosion residual areas (A10-A13), and the regions are composed not only by S, but also by Na. The atomic ratio of Na-to-S is approximately 1.4. According to the test result above, most Na breaks away from Na3AlF6 and complexes with S by atomic ratio of 1.4, thereby forming into a kind of stable structure during the carbon–air reaction. This stable structure lost the catalysis that they have had before, and the lost Na keeps the catalysis effect in coke in the form of Na0.5AlO.
According to the analysis of the test and experimental results above, the mechanism analysis of the mutual inhibition between Na and S on air reactivity is as follows.
1) If only Na3AlF6 is in coke, Na3AlF6 that exists on the surface of coke would transform into Na1.6AlO during the carbon–air reaction, which would make the catalysis effect on coke, which can be explained by the theory proposed by MOULIJN and KAPTEIJN [13]; Na has a stronger affinity to O2 in the air than C in the coke during the carbon–air reaction, thus Na1.6AlO is similar to a bridge that attracts more O2 to the surface of the coke and speeds up the reaction between C and O2.

Fig. 6 SEM images after carbon-air reaction:
Table 4 Element composition of areas A6-A13 in Fig. 6
2) When both S and Na exist in the coke, most Na breaks away from Na3AlF6 and complexes with S by the atomic ratio of 1.4, thereby forming a kind of stable structure. This stable structure lost the catalysis that they have had before, and the lost S and Na, which is in the form of Na0.5AlO, keep the catalysis effect in coke.
Under the influence of the two kinds of behavior above, the experiment results are as presented in Fig. 2.
3.2.2 Mechanism analysis of mutual inhibition between Na and S on CO2 reactivity
Figure 7 illustrates the SEM images and EDS analysis of sample NA6 after the CO2 reactivity tests. As shown in Fig. 7(b), the Na3AlF6 has been replaced by a new form of filamentous structure after carbon–CO2 reaction. The filamentous structures have C, O, Na, and Al elements, and the atomic ratio of Na-to-Al is approximately 4 (named Na4AlO). The filamentous structures mostly exist on the bottom and lining wall of the large corrosion holes, which are shown similar to the corrosion holes corroded by the filamentous structures (Fig. 7(c)). Na4AlO has a close relationship with the catalysis of Na to carbon–CO2 reaction.
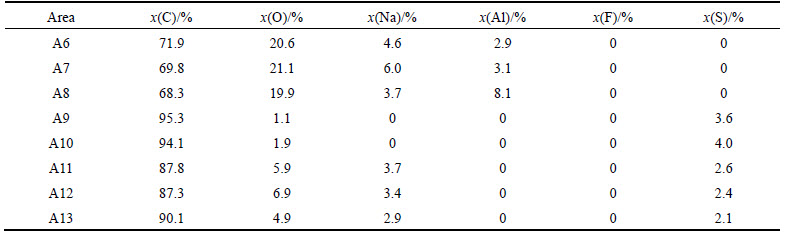
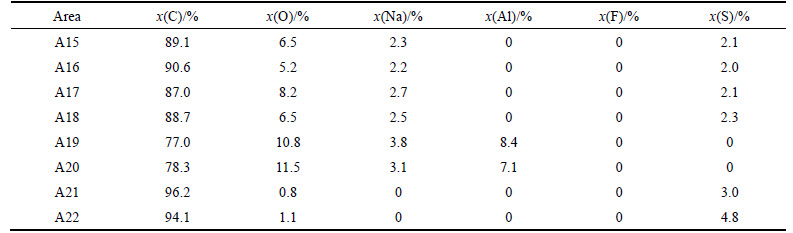
Figure 8 and Table 5 present the SEM images and EDS analysis of sample NSA6 after the CO2 reactivity tests. Two different kinds of white foreign particles exist on the surface. As shown in A15–A18 regions, this kind of foreign body has a regular shape structure and has C, O, Na, and S elements, and the atomic ratio of Na-to-S and Na-to-O is approximately 1.2 and 0.5, respectively (named as Na1.2SCO). As shown in Figs. 8(c)–(e), this kind of white foreign particle generally exists in the small corrosion grooves. Meanwhile, as shown in regions A19 and A20, other kinds of irregularly shaped structures exist, which have no S element, but with C, O, Na, and Al; the atomic ratio of Na-to-Al is <2, and Na-to-O ratio is high. Compared with A8 in Fig. 6(c), the irregularly shaped structures have the same element composition and element proportion with Na0.5AlO. Thus, the structures are speculated to be the same kind of matter; not similar to sample NAS6 after carbon–air reaction, no region where both S and Na are enriched together is observed. However, as shown in regions A21 and A22, S is enriched at some corrosion regions on the surface, therefore S still has catalysis effect on coke during carbon–CO2 reaction.
According to the analysis of the test and experimental results above, the inhibition of Na to the catalysis of S can affect the cyclic reaction system of S, in which Na1.2SCO should to be the key. Na1.2SCO must be combined with varieties of Na compounds. The S in the Na1.2SCO cannot have a stable high valence state, such as SO42-, S2O32-, because of the reducing system of carbon–CO2 reaction (1000 °C, atmosphere of CO/CO2, C-rich, less H2S and SO2). Therefore, only Na2SO3, Na2S, Na2Sy, and Na2CO3 may exist.
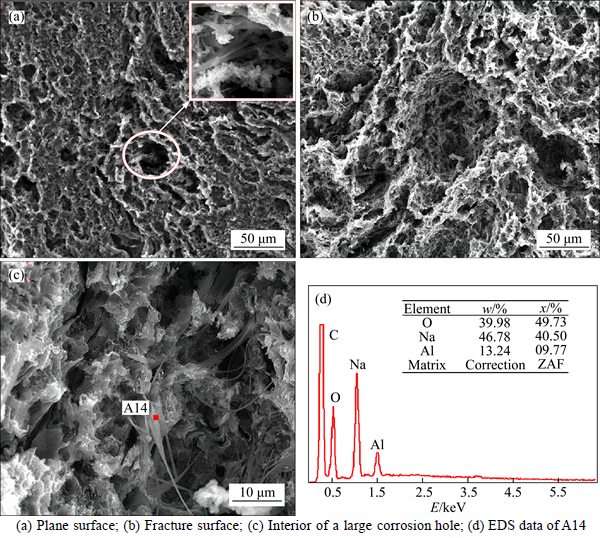
Fig. 7 SEM images and EDS data of NA6 after carbon-CO2 reaction:
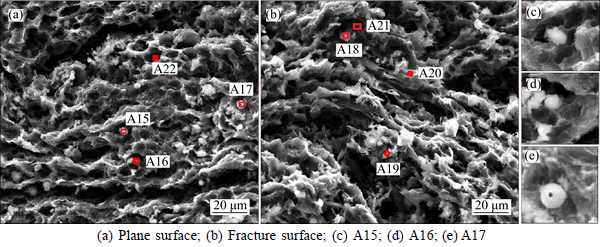
Fig. 8 SEM images and EDS data of NSA6 after carbon-CO2 reaction:
Table 5 Element composition of areas A15-A22 in Fig. 8
According to Ref. [14], the stability of Na2SO3 in the oxidizing atmosphere under high temperature is poor. At >600 °C, Na2SO3 decomposes into Na2S and Na2SO4 through reaction 1.
4Na2SO3=Na2S+3Na2SO4, △rG01000°C=-45.8 kJ/mol (1)
At >700 °C and carbon-rich condition, Na2SO4 transforms into Na2S via reaction 2. Reaction 2 is one of the main methods of Na2S preparation in the industry [15, 16].
Na2SO4+4C=Na2S+4CO(g), △rG01000°C=-236.2 kJ/mol (2)
Under carbon-rich and CO2 conditions, Na2CO3 rapidly transforms into Na2S through reaction 3 [17, 18].
Na2S+8CO2(g)+4C=Na2CO3+SO2(g)+11CO(g),
△rG01000°C=-80.9 kJ/mol (3)
In Refs. [19, 20], it was pointed out that Na2CO3 has strong adsorption ability to sulfurous gas by reactions 4 and 5, thereby generating Na2SO3 and Na2S.
Na2CO3+SO2(g)=Na2SO3+CO2(g),rG01000°C=-11.4 kJ/mol (4)
Na2CO3+H2S(g)=Na2S+CO2(g)+H2O(g),△rG01000°C=-31.2 kJ/mol (5)
Below 1200 °C by reaction 3 (react quickly below 800 °C), Na2Sy is generated in the presence of Na2S and elemental S[21].
Na2S+Sx→Na2Sy (x=1, 2, 4, 6, 8; y>1) (6)
In addition, [Na] can be seen as the active Na atom on coke surface. The initial generation process of Na2SO3 and Na2S cannot be speculated concretely, hence simplified into reactions 7 and 8.
[Na]+SO2(g)→Na2SO3 (7)
[Na]+H2S(g)→Na2S (8)
Therefore, combining the reactions above, the inhibition of Na to S during carbon–CO2 reaction is speculated to be generated by a set of reaction systems as followed from reactions 7 and 8 to reactions 1, 2, 3, 4, 5 and 6. With the situation of CO2 atmosphere and 1000 °C, the structures in coke containing Na and S elements cannot remain stable. Na and S come into contact with the outside atmosphere gradually. S begins to show its catalysis to carbon-CO2 reaction in the forms of H2S, SO2, COS and elemental S. At the same time, the Na element in coke changes into [Na]. [Na] will capture SO2 and H2S, and Na2SO3 and Na2S will generate. By reactions 1 and 2, volatile Na2SO3 will change into Na2S. By reaction 3, Na2S is transformed into Na2CO3, with releasing S element in the form of SO2. However, Na2CO3 will capture SO2 and H2S again, with generating Na2SO3 and Na2S (reactions 4 and 5). Therefore, the cyclic reaction system “Na2SO3→Na2S→Na2CO3→Na2SO3” consumes the SO2 and H2S continually, which could destroy the original catalytic cycle reaction system of S. When carbon-CO2 reaction is over, with the temperature reduction, Na2Sy will be formed by reaction 6. After cooling, the surface of coke sample will leave the mixture of Na2SO3, Na2S, Na2CO3 and Na2SO3.
However, a certain amount of S could still make catalytic effect on coke during carbon–CO2 reaction because of the large content difference between S and Na, which is also the reason why the carbon–CO2 reaction residue of high-sulfur coke cannot reach that of the low-sulfur coke. Furthermore, after carbon–CO2 reaction, Na0.5AlO-like analogues are found on the NAS6 surface. Thus, when the Na content exceeds 360×10-6, the CO2 residue of high-sulfur coke slightly decreases (Fig. 3), and the inhibition efficiency of Na to S is also limited during the carbon–CO2 reaction. With increased Na content until the limit, more Na becomes independence of S and keeps their catalysis to coke in the form of Na0.5AlO.
4 Conclusions
When both Na (Na3AlF6) and S impurities exist in coke, mutual inhibition occurs between the individual catalytic activities of S and Na. Na can effectively inhibit the catalysis of S during carbon–air/CO2 reactions, and S could inhibit the catalysis of Na during carbon–air reaction to a certain extent. Taken together, our results indicate that the mechanism of mutual inhibition between S and Na during carbon–air/CO2 reactions is as follows:
1) During carbon–air reaction, Na composites with S at 1.4 atomic ratio form a kind of stable structure. At the same time, the inhibition efficiency of Na to S is also limited. With increased Na content, Na partly regains its catalysis to coke through the form of Na0.5AlO.
2) During carbon–CO2 reaction, the inhibitive effect of Na on the reactive catalysis of S is most probably related to each other by the cyclic reaction system “Na2SO3→Na2S→Na2CO3→Na2SO3”, which could destroy the original catalytic cycle reaction system of S.
References
[1] ZHANG Jia-yuan, ZHOU Jie-min, YAN Hong-jie. Kinetic model on coke oven gas with steam reforming [J]. Journal of Central South University of Technology, 2008, 15(1): 127-131.
[2] AL-HAJ-IBRAHIM H, MORSI B I. Desulfurization of petroleum coke: A review [J]. Industrial & Engineering Chemistry Research, 1992, 31(8): 1835-1840.
[3] LEBRETON R, BRUNET S,  V, KASZTELAN S. Influence of sulfur containing compounds on high temperature coke formation on hydrotreating catalyst [J]. Studies in Surface Science and Catalysis, 2000, 130: 2861-2866.
V, KASZTELAN S. Influence of sulfur containing compounds on high temperature coke formation on hydrotreating catalyst [J]. Studies in Surface Science and Catalysis, 2000, 130: 2861-2866.
[4] XIAO Jin, ZHONG Qi-fan, DENG Song-yun, LAI Yan-qing, LI Jie, LIU Ye-xiang. Mechanism and effect of sulfur on coke air reactivity and mutual restrain between calcium and sulfur [J]. Journal of Central South University: Science and Technology, 2015, 46(10): 3603-3610. (in Chinese)
[5] GRIGORE M, SAKUROVS R, FRENCH D, SAHAJWALLA V. Coke gasification: The influence and behavior of inherent catalytic mineral matter [J]. Energy & Fuels, 2009, 23(3): 2075-2085.
[6] EIDET TRYGVE, SOERLIE M, LARSEN S K. The effect of sodium salts on binder coke reactivity [J]. Light Metals Warrendale, 1999: 523-530.
[7] MEIJER R, WEEDA M, KAPTEIJN F, MOULIJN JA. Catalyst loss and retention during alkali-catalysed carbon gasification in CO2 [J]. Carbon, 1991, 29(7): 929-941.
[8] KAPTEIJN F, PEER O, MOULIJN J A. Kinetics of the alkali carbonate catalysed gasification of carbon: 1. CO2 gasification [J]. Fuel, 1986, 65(10): 1371-1376.
[9] ENGVOLL M A. Reactivity of anode raw materials and anodes for production of aluminium [D]. Trondheim: Institute for Kjemi, 2002: 87-97.
[10] XIAO Jin, ZHONG Qi-fan, LI Fa-chuang, HUANG Jin-di, ZHANG Yan-bin, WANG Bing-jie. Mutual inhibition between catalytic impurities of sulfur and those of calcium in coke during carbon-air and carbon-CO2 reactions [J]. Energy & Fuels, 2015, 29(3): 1961-1971.
[11] XIAO Jin, DENG Song-yun, ZHONG Qi-fan, YE Shao-long. Effect of sulfur impurity on coke reactivity and its mechanism [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3702-3709.
[12] HUME S M, FISCHER W K, PERRUCHOUD R C, METSON J B, BAKER R T. Influence of petroleum coke sulphur content on the sodium sensitivity of carbon anodes [J]. Light Metals Warrendale, 1993: 535-535.
[13] MOULIJN J A, KAPTEIJN F. Carbon gasification reactions [M]. Sciences of Carbon Materials. Publicaciones da Universidad de Alicante, 2000: 379-402.
[14] WANG Zhen. Chemical dictionary [M]. Beijing: Chemical Industry Press, 2000. (in Chinese)
[15] ZHAO Xiu-ping. Study on the technology of white sodium sulphide crystal [J]. Inorganic Chemicals Industry, 2005, 37(4): 29-31. (in Chinese)
[16] LI Wen-xiu, YUN Chen, JIREN T, QIN Hai-li, ZHENG Hong-na. Preparation of sodium sulfide from reduction of sodium sulfate by coke oven coal gas [J]. Journal of Inner Mongolia Polytechnic University: Natural Science, 2006, 24(2): 109-113. (in Chinese)
[17] ZHOU Chang-sheng. Study and improvement of the production process of sodium sulphide [J]. Chemical Engineering (China), 2005, 33(1):75-78. (in Chinese)
[18] DAI Cheng-zhi, ZHANG Shou-tang, ZHAO Guang-ning, YANG Jian-shan, YU Xin-hong, ZHENG Zhi-yong, LIU Jian-hua. Production practice of white flake shape industrial sodium sulfide [J]. Inorganic Chemicals Industry, 2005, 37(7): 36-39.
[19] ZHANG Guo-qing, CHEN Yan-hua. Preliminary analysis on Na2CO3 absorption mechanism in PDS desulfurization technology [J]. Fuel & Chemical Processes, 2008, 39(5): 32-34.
[20] ZESILAR R, HE Yu-xiu. Desulfurization of coal gas by sodium carbonate catalytic method [J]. Fuel & Chemical Processes, 2002, 5: 023-027.
[21] LI Jing-ping, CAI Xin-an, ZHANG Hui-fang, CHENG Xu-jun, WANG Kai-kai1. Prepare sodium polysulfide with coking black sulfur [J]. Chemical Engineer, 2012, 31(7): 56-57. (in Chinese)
(Edited by YANG Hua)
Cite this article as: ZHONG Qi-fan, XIAO Jin, LI Fa-chuang, LI Jie. Inhibition mechanism between sodium (Na3AlF6) and sulfur on coke reactivity [J]. Journal of Central South University, 2017, 24(8): 1736-1744. DOI: https://doi.org/ 10.1007/s11771-017-3581-y.
Foundation item: Projects(51374253, 51574289) supported by the National Natural Science Foundation of China
Received date: 2016-02-29; Accepted date: 2016-05-23
Corresponding author: XIAO Jin, Professor, PhD; Tel: +86-731-88830277; E-mail: changshaxiaojin@126.com

