文章编号:1004-0609(2007)10-1705-06
室温固相反应-煅烧分解法制备镍锰氧化物
杨 波,王志兴,郭华军,李新海,周友元
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:以NiCl2?6H2O和MnCl2?4H2O为主盐,分别与(NH4)2C2O4?H2O、NaOH和Na2CO3?10H2O经室温固相合成相应的镍锰化合物,然后分别在400、300、500 ℃温度下煅烧分解3 h得到镍锰氧化物。结合已知的热力学数据,对室温固相反应的吉布斯自由能变和焓变进行计算,从热力学的角度出发,研究不同的反应类型对产物结构的影响。采用DSC-TGA、XRD等方法对煅烧分解过程、氧化物的物相和微观形貌进行了分析与表征。结果表明:镍锰草酸盐分解得到NiMn2O4和MnNi2O4;镍锰氢氧化物分解只得到MnNiO3;镍锰碳酸盐分解得到NiMn2O4、MnNi2O4和MnNiO3。镍锰氧化物粒径依次增大的顺序为:镍锰氢氧化物、镍锰草酸盐、镍锰碳酸盐的产物。
关键词:镍锰氧化物;固相反应;热力学分析
中图分类号:TM 912.9 文献标识码:A
Preparation of nickel-manganese composite oxide by room temperature solid state reaction-calcining decomposition
YANG Bo, WANG Zhi-xing, GUO Hua-jun, LI Xin-hai, ZHOU You-yuan
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Using NiCl2?6H2O and MnCl2?4H2O as major reactants, nickel-manganese composites were synthesized by the solid state reaction at room temperatures with three difference reactants ((NH4)2C2O4?H2O, NaOH, NaCO3?10H2O). The nickel-manganese oxides were synthesized by calcining three precursors at 400, 300, 500 ℃ for 3 h.  and
and  for three solid state reactions at room-temperature were estimated by the thermodynamic data. The effects of difference reaction on the oxides were studied. The processes, particle size and crystallography characters were studied by means of DSC-TGA, XRD and TEM. The results show that spinel NiMn2O4 and MnNi2O4 are obtained by decomposing nickel-manganese oxalic acid; Ilmenite-type MnNiO3 is obtained by decomposing nickel-manganese hydroxide; Ilmenite-type MnNiO3, spinel NiMn2O4 and MnNi2O4 are obtained by decomposing nickel-manganese carbonate. The particle size of nickel-manganese oxide products increases by turn of nickel-manganese hydroxide, oxalic acid, carbonate.
for three solid state reactions at room-temperature were estimated by the thermodynamic data. The effects of difference reaction on the oxides were studied. The processes, particle size and crystallography characters were studied by means of DSC-TGA, XRD and TEM. The results show that spinel NiMn2O4 and MnNi2O4 are obtained by decomposing nickel-manganese oxalic acid; Ilmenite-type MnNiO3 is obtained by decomposing nickel-manganese hydroxide; Ilmenite-type MnNiO3, spinel NiMn2O4 and MnNi2O4 are obtained by decomposing nickel-manganese carbonate. The particle size of nickel-manganese oxide products increases by turn of nickel-manganese hydroxide, oxalic acid, carbonate.
Key words: nickel-manganese oxide; solid state reaction; thermodynamic analysis
自从2001年日本东京工业大学Ohzuku[1]首次合成锂离子二次电池正极材料层状LiNi0.5Mn0.5O2以来,LiNi0.5Mn0.5O2以可逆容量高、循环性好、安全性高、成本低廉等优点受到了众多研究者的关注[2-6]。Yasuhiro等[7]提出镍锰氧化物可以作为锂镍锰复合氧化物合成中的重要原料,引起了广泛的关注。其合成方法、结构、性能和应用的研究成为近年来研究的热点之一[8-10]。室温固态化学反应是20世纪80年代末发展起来的一种新的合成方法,其最大的特点在于反应温度降至室温或近室温,因而具有便于操作和控制的优点。此外还有不使用溶剂、高选择性、高产率、污染少、节省能源、合成工艺简单等特点,这些特点符合当今社会绿色化学发展的要求[11]。因此本文作者针对LiNi0.5Mn0.5O2的原料问题,通过室温固相法,以NiCl2?6H2O和MnCl2?4H2O为主盐,与不同的反应物反应合成不同的镍锰化合物,然后煅烧分解得到相应的镍锰氧化物。所采用的室温固相法方法简单,有利于研究不同种类的反应物对镍锰氧化物合成的影响,从而筛选出合适的反应物来合成镍锰离子均匀混合的复合氧化物,作为层状LiNi0.5Mn0.5O2的制备原料。在固相化学反应中,反应能否进行,取决于固体反应物的结构和热力学性质。在满足热力学条件下,固体反应物的结构决定了固相反应进行的速率[11]。本研究从热力学的角度出发,研究不同的反应类型对产物的影响。采用DSC-TGA、XRD、SEM等方法对合成的镍锰氧化物的物相和微观形貌进行了分析与表征。
1 实验
1.1 镍锰氧化物的制备
采用的试剂NiCl2?6H2O,MnCl2?4H2O,(NH4)2C2O4?H2O,NaOH,Na2CO3?10H2O均为分析纯。其中,主盐NiCl2?6H2O、MnCl2?4H2O分别以N、M标记,反应物(NH4)2C2O4?H2O、NaOH、NaCO3?10H2O分别以1、2、3标记。
在室温条件下,将N、M按反应配比1?1(摩尔比)在玛瑙研钵中研磨混合均匀后,再按N:M:X的反应摩尔比1?1?2.4加入X(X为反应物1、2、3中的一种,当X为NaOH时,反应摩尔比为1?1?4.8)。混匀后,进行匀速研磨,充分研磨30 min。反应物释放出结晶水,使反应物逐步变成稠状湿润的固体。其中,合成镍锰草酸盐时,Cl-以NH4Cl的形式存在,可在后期煅烧分解时升华成气体除去,故不需经过水洗步骤,将研磨所得产物直接干燥得镍锰草酸盐前驱体;合成镍锰氢氧化合物和镍锰碳酸盐时,为去除产物中所含Cl-,研磨反应完全后,在超声波作用下用去离子水洗涤、过滤,洗涤多次后用AgNO3检测,直至无Cl-为止。然后用无水乙醇洗涤,60 ℃下干燥得到相应的前驱体。将干燥后的各前驱体在各自的分解温度下煅烧3 h,得到镍锰氧化物。
1.2 性能表征
采用SDT-Q600热分析仪(英国),在空气气氛中对前驱体进行DSC-TGA分析:升温速度10 ℃/min;用Rigaku D/max2550VB+18KW转靶X射线衍射仪(日本)分析样品物相,Cu Kα辐射,40 kV,电流50 mA,步宽0.02 (?),扫描速度2 (?)/min,扫描范围2θ为10?~90?;用日本JEOL公司的JSM-5600 LV扫描电子显微镜对样品的表面形貌进行观察。
2 结果与讨论
2.1 室温固相反应的热力学计算
固相化学反应和溶液中的化学反应一样,必须遵循热力学的规律,即整个反应的吉布斯自由能变小于零。反应系统中各物质的标准生成吉布斯自由能 和标准焓
和标准焓 ,可以查表获得,再根据以下公式可以计算出指定反应的吉布斯自由能变和焓变。
,可以查表获得,再根据以下公式可以计算出指定反应的吉布斯自由能变和焓变。

在环境温度为25~30 ℃的情况下,将各室温固相反应系统物质的吉布斯自由能和焓近似为标准生成吉布斯自由能和标准焓,可计算出各反应的吉布斯自由能变和焓变。
由表1数据,计算各反应式及吉布斯自由能变和焓变结果如下:
NiCl2?6H2O+(NH4)2C2O4→NiC2O4+2NH4Cl+6 H2O
 = -13.82 kJ/mol<0
= -13.82 kJ/mol<0
 = -29.37 kJ/mol<0
= -29.37 kJ/mol<0
MnCl2?4H2O+(NH4)2C2O4→MnC2O4+2NH4Cl+4 H2O
 = -34.59 kJ/mol<0
= -34.59 kJ/mol<0
 = -34.14 kJ/mol<0
= -34.14 kJ/mol<0
NiCl2?6H2O+2NaOH→Ni(OH)2+2NaCl+6H2O
 =-165.44 kJ/mol<0
=-165.44 kJ/mol<0
 = -112.55 kJ/mol<0
= -112.55 kJ/mol<0
MnCl2?4H2O+2NaOH→Mn(OH)2+2NaCl+4H2O
 = -148.98 kJ/mol<0
= -148.98 kJ/mol<0
 = -206.02 kJ/mol<0
= -206.02 kJ/mol<0
NiCl2?6H2O+Na2CO3?10H2O→NiCO3+2NaCl+16 H2O
 = -33.35 kJ/mol<0
= -33.35 kJ/mol<0
 =150.92 kJ/mol>0
=150.92 kJ/mol>0
MnCl2?4H2O+Na2CO3?10H2O→MnCO3+2NaCl+14H2O
表1 反应物与生成物在298.15 K下的热力学数据[13]
Table 1 Thermodynamic data of reactants and products at 298.15 K

 = -48.27 kJ/mol<0
= -48.27 kJ/mol<0
 = 62.05 kJ/mol>0
= 62.05 kJ/mol>0
由计算结果可知,在室温下,上述各反应的 均小于零,故反应正向进行。其中,NiCl2?6H2O、MnCl2?4H2O和(NH4)2C2O4?H2O或NaOH的反应为放热反应(
均小于零,故反应正向进行。其中,NiCl2?6H2O、MnCl2?4H2O和(NH4)2C2O4?H2O或NaOH的反应为放热反应(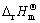 <0),NiCl2?6H2O、MnCl2?4H2O和Na2CO3?10H2O的反应为吸热反应(
<0),NiCl2?6H2O、MnCl2?4H2O和Na2CO3?10H2O的反应为吸热反应(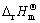 >0)。在室温固相反应中,反应物通过研磨进行机械活化,反应物具有较高的表观活化能,反应物互相接触后极易发生反应。由于产物晶体结构不同于反应物,生成的产物很快从反应物团聚体上脱落下来,内层的反应物继续得到机械活化,从而反应不断地进行,直至反应完全。
>0)。在室温固相反应中,反应物通过研磨进行机械活化,反应物具有较高的表观活化能,反应物互相接触后极易发生反应。由于产物晶体结构不同于反应物,生成的产物很快从反应物团聚体上脱落下来,内层的反应物继续得到机械活化,从而反应不断地进行,直至反应完全。
2.2 镍锰前驱体的DSC-TGA分析
图1(a)所示为镍锰草酸盐前驱体的DSC-TGA曲线。镍锰草酸盐前驱体的质量损失曲线分3段。第1段对应温度较低(低于160 ℃),由草酸铵((NH4)2C2O4?H2O)加热至95 ℃时脱水,加高热即分解的性质[13]可知:此段应为吸附水的失去和反应剩余的过量草酸铵的分解,质量损失率为7.59%,与理论质量损失率7.84%接近,对应一个吸热峰;第2段对应温度为160~300 ℃,可归结为镍锰草酸盐分解为镍锰氧化物,质量损失率为43.15%,与理论质量损失率40.46%接近,对应一个吸热峰;第3段对应温度为300~400 ℃,由于氯化铵的升华温度为340 ℃,此时质量损失可认为主要是氯化铵升华所造成,质量损失率为21.19%,理论值为17.23%。图1(b)所示为镍锰氢氧化物前驱体的DSC-TGA曲线。由图可见,300 ℃以前,样品持续分解,伴随一个很小的吸热峰,质量损失率约为4.88%,此过程估计是镍锰氢氧化物前驱体失去结晶水,分解为镍锰氧化物。图1(c)所示为镍锰碳酸盐前驱体的DSC-TGA曲线,质量损失曲线分段不明显,在155 ℃以前有15.34%的质量损失,此段应为吸附水的失去;155~500 ℃之间有两个较明显的吸热峰,分别对应质量损失率18.66%和5.8%,此段应为镍锰碳酸盐的分解;500 ℃后质量损失曲线趋于水平,无明显质量损失。

图1 不同镍锰化合物的DSC-TGA图
Fig.1 DSC-TGA curves of nickel-manganese composites: (a) Oxalic acid; (b) Hydroxide; (c) Carbonate
从图1可以看出,镍锰草酸盐在400 ℃、镍锰氢氧化物在300 ℃、镍锰碳酸盐在500 ℃基本分解完全。因此将镍锰草酸盐、镍锰氢氧化物和镍锰碳酸盐分别在上述分解温度下煅烧3 h,得到相应的镍锰氧化物。
2.3 煅烧分解后产物特征
图2所示为镍锰草酸盐在400 ℃、镍锰氢氧化物在300 ℃、镍锰碳酸盐在500 ℃分别煅烧分解3 h后所得的镍锰氧化物的SEM像。从图中可看出,镍锰草酸盐煅烧分解后产物外观呈絮状,粒径大小为50 nm左右,镍锰氢氧化物分解后产物颗粒较细,粒径大小为20 nm左右;镍锰碳酸盐分解后产物近似球形,粒径大小为200 nm左右。

图2 煅烧分解后的镍锰氧化物的SEM像
Fig.2 SEM images of Ni-Mn composite oxides obtained by decomposition of nickel-manganese oxalic acid (a), hydroxide (b) and carbonate (c)
经室温固相反应合成的各前驱体煅烧分解后生成的产物中仅有Ni、Mn两种金属元素。酸溶氧化物后,经丁二酮肟质量分析测得Ni元素的含量,再经EDTA化学滴定分析样品中Ni、Mn的总含量,于是得到Mn元素的含量。镍锰氧化物的镍锰质量分数及摩尔比列于表2。
表2 不同镍锰氧化物的镍锰质量分数和摩尔比
Table 2 Nickel and manganese percentage of different Ni-Mn composite oxides and molar ratio
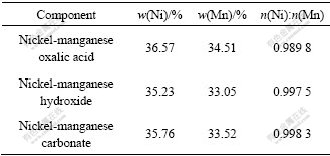
图3所示分别为镍锰草酸盐、镍锰氢氧化物和镍锰碳酸盐煅烧分解后所得的镍锰氧化物的XRD谱。镍锰草酸盐分解产物(图3(a))与尖晶石型NiMn2O4(标准JCPDS卡01-1110)和反尖晶石型MnNi2O4(标准JCPDS卡36-0083)峰位置与强度基本一致。由于尖晶石型NiMn2O4和反尖晶石型MnNi2O4的峰的位置基本重合,强度相差不大,故镍锰草酸盐分解产物可能含有NiMn2O4和MnNi2O4两种物相,由表2中不同前驱体煅烧分解后所得镍锰氧化物的镍锰百分含量及摩尔比可知,镍锰草酸盐前驱体煅烧分解所得的产物中镍锰摩尔比与反应前原料的投料比1?1基本一致,故产物中NiMn2O4和MnNi2O4两种物相共存;镍锰氢氧化物分解后得到的产物(图4(b))的峰位置与强度均与钛铁矿型MnNiO3(标准JCPDS卡65-3695)的数据基本一致,但产物的特征衍射峰宽度较大,而且强度较弱,根据X射线多晶衍射理论,说明固相产物的粒度较小,晶型较差;镍锰碳酸盐分解后得到NiMn2O4、MnNi2O4和MnNiO3三种物相(图3(c))。根据XRD谱的峰宽计算得图3(a)和图3(b)所代表的镍锰氧化物的晶粒尺寸分别为56 nm和13.8 nm,与SEM像的结果相对应。

图3 不同的镍锰氧化物的XRD谱
Fig.3 XRD patterns of Ni-Mn composite oxides obtained by decomposition of nickel-manganese oxalic acid (a), hydroxide (b) and carbonate (c)
XRD的数据表明,镍盐、锰盐与NaOH反应得到的前驱体经煅烧分解可形成MnNiO3单一物质,镍锰原子混合均匀。三个不同的反应类型生成单相或多相物质,其原因可能在于主盐NiCl2?6H2O和MnCl2?4H2O与NaOH在室温固相反应时生成了水合镍锰复合氧化物,镍锰两种离子作为一种化合物中的成分共存于水合镍锰复合氧化物中,达到了镍离子与锰离子混合均匀的目的,后经煅烧分解,生成了单一相MnNiO3。通过对比镍锰氢氧化物前驱体的XRD谱(见图4)与程锦国[15]用共沉淀所合成的水合镍锰复合氧化物NiMnO3?H2O的XRD谱可以发现两者的主峰位置、强度基本一致,证明了该观点。主盐NiCl2?6H2O、MnCl2?4H2O在与Na2CO3?10H2O反应时,由于反应发生后,存在大量的生成水,整个反应体系可以近似于液相反应体系,Ni2+和Mn2+两种离子可以视为在溶液里发生了共沉淀。可是由于NiCO3和MnCO3的溶度积相差较大,NiCO3的溶度积为6.6×10-9,MnCO3的溶度积为1.8×10-11[16],因此造成Ni2+和Mn2+两种离子在共沉淀时生成的产物并不是按照1?1的比例均匀沉淀的,故煅烧分解后产物的物相有3种:NiMn2O4、MnNi2O4和MnNiO3,且由XRD谱可知,MnNiO3峰的强度较强,镍锰碳酸盐煅烧分解后的产物主要为MnNiO3。而主盐NiCl2?6H2O和MnCl2?4H2O与(NH4)2C2O4?H2O反应所生成的水有限,Ni2+和Mn2+两种离子主要是在固相界面上发生接触,镍锰两种离子没有达到原子水平的均匀混合,混合程度最差,故煅烧分解后生成NiMn2O4和MnNi2O4两种物相。

图4 镍锰氢氧化物前驱体的XRD谱
Fig.4 XRD pattern of nickel-manganese hydroxide precursor
3 结论
1) 通过对以NiCl2?6H2O和MnCl2?4H2O为主盐,与(NH4)2C2O4?H2O、NaOH和Na2CO3?10H2O的室温固相反应进行热力学计算可知,反应均可正向进行,其中与(NH4)2C2O4?H2O或NaOH的反应为放热反应,与Na2CO3?10H2O的反应为吸热反应。
2) 在以上3个室温固相反应中,与NaOH反应生成的前驱体煅烧分解后所得的镍锰氧化物颗粒最细,与(NH4)2C2O4?H2O反应生成的前驱体煅烧分解后所得的产物颗粒次之,与Na2CO3?10H2O反应生成的前驱体煅烧分解后所得产物颗粒最粗。
3) XRD研究表明,镍锰草酸盐分解得到NiMn2O4和MnNi2O4,镍锰氢氧化物分解得到MnNiO3,镍锰碳酸盐分解得到NiMn2O4、MnNi2O4和MnNiO3的混合物相。
4) 从产物的成分、形貌结构考虑,主盐NiCl2?6H2O和MnCl2?4H2O与NaOH经室温固相反应-煅烧分解法合成的MnNiO3性能最好,可作为锂离子二次电池正极材料LiNi0.5Mn0.5O2的原料。
REFERENCES
[1] Ohzuku T, Makimura Y. Layered lithium insertion material of LiNi0.5Mn0.5O2: A possible alternative to LiCoO2 for advanced lithium-ion batteries[J]. Chem Lett, 2001, 8: 744-745.
[2] Lu Z H, Macneil D D, Dahn J R. Layered cathode materials Li[Ni, Li(1/3-2x/3)Mn(2/3-x/3)]O2 for lithium-ion batteries[J]. Electrochemical and Solid-States Letters, 2001, 4(11): A191-A194.
[3] Saifu I M, Andrew D R. Structural and electronic properties of the layered LiNi0.5Mn0.5O2 lithium battery material[J]. Journal of Chemistry Materials, 2003, 12: 4280-4286.
[4] Yoon W S, Grey C P. In situ X-ray absorption spectroscopic study on LiNi0.5Mn0.5O2 cathode material during electrochemical cycling[J]. Journal of Chemistry Materials, 2003, 15(16): 3161-3169.
[5] Reed J, Ceder G. Charge, Potential, and phase stability of layered Li(Ni0.5Mn0.5)O2[J]. Electrochemical and Solid State Letters, 2002, 5(7): A145-A148.
[6] 肖 劲, 曾雷英, 彭忠东, 赵 浩, 胡国荣. 锂离子电池正极材料LiNi0.5Mn0.5O2的循环性能[J]. 中国有色金属学报, 2006, 16(8): 1439-1445.
XIAO Jin, ZENG Lei-ying, PENG Zhong-dong, ZHAO Hao, HU Guo-rong. Cycle performance of LiNi0.5Mn0.5O2 as cathode material for lithium secondary batteries[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(8): 1439-1445.
[7] Yasuhiro F, Hiroshi M. Lithium nickel manganese mixed oxides, their manufacture, and lithium ion secondary battery using them[P]. JP 2004 002141, 2004.
[8] Kovanda F, Grygar T, Dornicak V. Thermal behaviour of Ni-Mn layered double hydroxide and characterization of formed oxides[J]. Solid State Sciences, 2003, 5(7): 1019-1026.
[9] Gyrdasova O I, Bazuev G V, Krasilpnikov V N, et al. Synthesis of NiMnO3 ilmenite by thermal decomposition of double oxalates and their hydrazine compounds[J]. Journal Neorganicheskoi Khimii, 2003, 48(9): 1446-1451.
[10] Mehandjiev D, Zhecheva E, Ivanov G. Preparation and catalytic activity of nickel-manganese oxide catalysts with an ilmenite-type structure in the reactions of complete oxidation of hydrocarbons[J]. Applied Catalysis, A: General, 1998, 167(2): 277-282.
[11] 杨 彧, 贾殿赠, 葛炜炜, 金春飞, 忻新泉. 低热固相反应制备无机纳米材料的方法[J]. 无机化学学报, 2004, 8(8): 881-888.
YANG Yu, JIA Dian-zeng, GE Wei-wei, JIN Chun-fei, XIN Xin-quan. Synthetic of inorganic nano-materials by solid state reaction at low-heating temperature[J]. Wuji Huaxue Xuebao(Chinese J Inorg Chem), 2004, 8(8): 881-888.
[12] 谭 倪, 王凤洁. 固相化学反应的热力学分析及其优势探讨[J]. 郴州师范高等专科学校学报, 2003, 24(5): 68-70.
TAN Ni, WANG Feng-jie. Analyzing the thermodynamics of solid chemical reaction and making an inquiry into its superiority[J]. Journal of Chenzhou Teachers College (Chinese J Inorg Chem), 2003, 24(5): 68-70.
[13] 迪安 J A. 兰氏化学手册[M]. 尚久方, 译. 北京: 科学出版社, 1985: 1470-1520.
Dean J A. Lange’s handbook of chemistry[M]. SHANG Jiu-fang, transl. Bejing: Science Press, 1985: 1470-1520.
[14] 杨显万, 何蔼平. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社 1985: 429.
YANG Xian-wang, HE Ai-ping. Calculating handbook of thermodynamic data in high temperature solution[M]. Beijing: Metallurgical Industry Press, 1985: 429.
[15] 程锦国, 梁永光, 王驰伟. 共沉淀法合成一种新型的水合镍锰复合氧化物NiMnO3?H2O[J]. 化学与生物工程, 2005, 22(8): 16-18.
CHENG Jin-guo, LIANG Yong-guang, Wang Chi-wei. Synthesis of a novel nickel manganese oxide hydrate-NiMnO3?H2O by coprecipitation method[J]. Chemistry and Bioengineering, 2005, 22(8): 16-18.
[16] 梁英教, 车荫昌. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社, 1993: 618.
LIANG Ying-jiao, CHE Yin-chang. Handbook of inorganic substance thermodynamics parameter[M]. Shenyang: Northeastern University Press, 1993: 618.
收稿日期:2007-02-27;修订日期:2007-06-06
通讯作者:王志兴,教授;电话:0731-8836633;E-mail: zxwang@mail.csu.edu.cn
(编辑 何学锋)