
Effects of electrolytes variation on formation of oxide layers of
6061 Al alloys by plasma electrolytic oxidation
Kai WANG1, Bon-Heun KOO1, Chan-Gyu LEE1, Young-Joo KIM1, Sung-Hun LEE2, Eungsun BYON2
1. School of Nano & Advanced Materials Engineering, Changwon National University,
Changwon, 641-773, Korea;
2. Surface Technology Research Center, Korea Institute of Materials Science,
Changwon, 641-010, Korea
Received 18 June 2008; accepted 10 March 2009
Abstract: Plasma electrolytic oxidation(PEO) processes were carried out to produce ceramic layers on 6061 aluminum substrates in four kinds of electrolytes such as silicate and aluminate solution with and without sodium fluorosilicate. The PEO processes were carried out under a hybrid voltage (260 V DC combined with 200 V, 60 Hz AC amplitude) at room temperature for 5 min. The composition, microstructure and element distribution analyses of the PEO-treated layers were carried out by XRD and SEM & EDS. The effect of the electrolyte contents on the growth mechanism, element distribution and properties of oxide layers were studied. It is obvious that the layers generated in aluminate solutions show smoother surfaces than those in silicate solutions. Moreover, an addition of fluorine ion can effectively control the layer porosity; therefore, it can enhance the properties of the layers.
Key words: plasma electrolytic oxidation; oxide layer; Al alloy; alkaline electrolyte
1 Introduction
Recently a number of deposition techniques, such as arc-discharge plasma, gas-flame spray, vacuum deposition and high temperature glass enameling, have been investigated to produce layers on light alloys, to increase their wear resistances, corrosion resistance, and so on. However, these techniques require high-energy consumptions to provide adequate layers adhesion under high contact loads. Therefore, a novel electrochemical surface treatment process, plasma electrolytic oxidation (PEO), was introduced, which could generate ceramic layers on light metals with low energy consumption[1]. Furthermore, researches showed that the PEO ceramic layers offered attractive combination of wear resistance, corrosion resistance, mechanical strength, interfacial adhesion and thermal property[2-4]. Indeed, practical applications of PEO process in the machine related industry have been discussed in Refs.[5-6].
PEO processes were typically carried in alkaline electrolytes containing silicate anions, which is environmental friendly and most widely used in earlier studies[7-8]. However, the influence of silicate and aluminate electrolyte systems on the microstructure and composition of PEO layers has rarely been considered and optimized. Especially, the exact way in which silicon and aluminate elements participate in formations of layers is less developed. Moreover, the electrolytes require to be optimized to fulfill the demands from industrial applications.
In the present work, the effect of silicate and aluminate alkaline electrolytes on the structure and composition of oxide layer formed by PEO process was studied. The surface morphology, microstructures, elements distribution and microhardness of ceramic layers were investigated.
2 Experimental
6061 Al alloy (Mg 1%; Si 0.65%; Fe 0.7%, Cu 0.3%; Cr 0.2%; Mn 0.15%; Ti 0.15%; and Al balance; mass fraction) with dimensions of 30 mm (diameter)× 16 mm (height) and surface roughness of Ra≤0.1 μm was used as a substrate. Al alloys were pretreated (degreased and ultrasonically cleaned in the acetone medium and thoroughly dried), and then immersed in electrolyte for PEO treatment. There were four kinds of electrolytes used in the experiment, which can be divided into silicate part and aluminate part. Specimens were PEO treated through four different electrolytes as shown in Table 1. During the treatment, the PEO process was carried out at the room temperature for 5 min in a 2 L water-cooled stainless steel tank, which also served as a counter electrode. As the anode, Al substrate was immersed in the electrolyte. According to previous research, a hybrid voltage of 200 V AC (60 Hz) with 260 V DC was applied from the power supply. After the PEO treatment, the specimens were thoroughly rinsed in running demineralized water and dried with compressed air.
Table 1 Compositions of experimental electrolytes

At first, the AC amplitude was slowly increased to 200 V to maintain the current density at 0.3-0.4 A/cm2 throughout the experimentation, afterwards the DC value was increased gradually till 260 V so as to maintain the pre-determined current density even as the layer thickness gradually increased. The plasmas would initiate to discharge on the surface at DC 140 V based on AC 200 V. During the PEO process, plenty amount of water vapor was generated by the exothermal PEO reaction.
The phases of the oxide layers were investigated with Philips-X’Pert system X-ray diffractometer(XRD) (Cu Kα radiation) and the scans were performed with 0.02? step size in the 2θ range of 30?-90?. The microstructures of surfaces and cross-sectional morphologies of PEO-treated samples were examined by a JSM 5610 scanning electron microscope(SEM). The layer element distribution was investigated by Oxford 6587 energy dispersive spectrometer(EDS). The microhardnesses of the layers at 10 different places were measured by VLPAK2000 Mitutoyo Hardness Test Machine using 0.1 N loading energy in 30 s dwelling time, and then the average microhardness was calculated.
3 Results and discussion
Fig.1 illustrates the surface features of the PEO- treated samples in four different kinds of electrolytes. It is clearly indicated that discharge channels in all electrolytes appear as the same dark circular spots, with pancake-like microstructures distributing all over the surface of the layers. It is also apparent that the layer surfaces treated in aluminate electrolytes are much smoother than those in silicate electrolytes. Because of the participation of silicon ions in the PEO reaction, the layers surfaces become more porous. Furthermore, by comparing A1, A2 and S1, S2 in Fig.1, the addition of Na2SiF6 affects the surface roughness. Actually, it even decreases the diameters of the discharging channels by introduction of fluorine ion, which is very effective to control the porosity of ceramic layers. X-ray diffraction patterns of PEO layers produced by the four processes are shown in Fig.2. Strong diffraction peaks of the Al substrate are detected because the layers are so thin and porous that X-rays can penetrate easily. It is shown that typical phase composition of PEO layers represented by α-Al2O3 and γ-Al2O3 can be found in the XRD spectrum. Moreover, mullite (Al2O3?nSiO2), an important ceramic phase characterized by good thermal and chemical stabilities, is also detected in silicate electrolytes (both in S1 and S2 processes). It is well known that trigonal α-Al2O3 is a stable alumina phase with a high melting point of 2 050 ℃, and γ-Al2O3 is a meta-stable phase which will transform into α-Al2O3 by heating up to the temperature interval between 800 and 1 200 ℃[9-10]. Before plasma discharge, a thin γ-Al2O3 film is generated first during the initial oxidation stage. McPHERSON[11] found that a high cooling rate favors the formation of α-Al2O3 during the solidification of alumina droplets in his study regarding the formation mechanism of meta-stable alumina phase for the plasma spraying technique. During the plasma electrolytic process, the molten γ-Al2O3 is injected to the electrolyte solution through the discharging channel when plasma sparks are produced, so γ-Al2O3 contacted with the electrolyte is quenched in a high cooling rate, which favors the formation of α-Al2O3 outside.
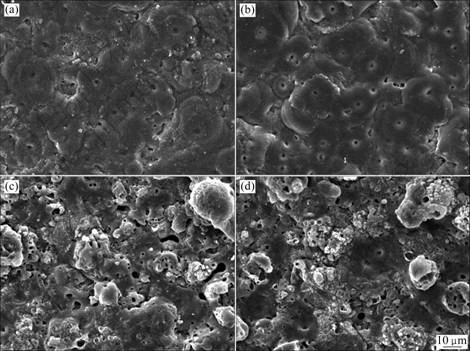
Fig.1 SEM surface morphologies of PEO-treated samples prepared in four kinds of electrolytes: (a) A1; (b) A2; (c) S1; (d) S2
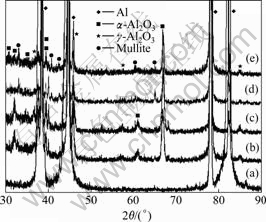
Fig.2 XRD patterns of ceramic layers prepared in four kinds of electrolytes: (a) Matrix; (b) A1; (c) A2; (d) S1; (e) S2
Ceramic layer surface treated by S2 electrolyte was investigated using EDS (sputtered with gold previously). The EDS results exhibit the element distribution in and around the discharged channel, as shown in Fig.3. From this figure, it is clear that Al content in the discharged channel is obviously much higher than that in the particles around it, while Si content in the particles is richer than that in the discharged channel. In silicate electrolytes, SiO32- generates SiO2 or SiO2·nH2O easily on the interface between layer and electrolyte. The molten alumina ejected out from the discharging channels where the temperature was so high (about 2×103-3×103 ℃[6]), will combine with SiO2 compound to form the mullite phases (Al2O3·nSiO2, see XRD patterns in Fig.2). By the force of plasma sparks, the mullite is punched around the discharging channel, and suddenly solidifies after encountering with the cold electrolytes. The mullite phases are easily turned to be small particles by the effects of surface tension. Finally, the mullite particles accumulate around the discharged channels, resulting in the volcano-like morphologies on the layer surfaces. In the A1 and A2 electrolyte, the centre of each pancake represents the discharging channel and is surrounded by circular rings of rapidly solidified molten alumina.
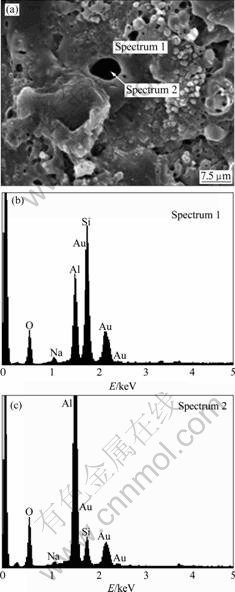
Fig.3 EDS analysis results on ceramic layer surface by S2 electrolyte: (a) Morphology; (b) and (c) EDS spectra
The cross-sectional SEM micrographs of the layers formed in four different kinds of electrolytes are shown in Fig.4. It is obvious that the samples prepared in silicate electrolytes (S1 and S2) present thicker layers than those prepared in aluminate electrolytes (A1 and A2). Na2SiF6 was added in A2 and S2 process as a special agent. It is easily decomposed to Si4+ and F-, where silicon ion is good for the growth of layers and fluoride ion is characterized as complex behavior with a wide interval of passivation[6] and good layer densities control ability (formation of tiny disperse Al2O3·xFx phase)[12]. It is apparent that the ceramic layers in A2 and S2 are thicker and denser than the layers in A1 and S1. This proves that Na2SiF6 can effectively enhance growth ability and density of the ceramic layers.

Fig.4 Cross-sectional SEM micrographs of layers prepared in four kinds of electrolytes: (a) A1; (b) A2; (c) S1; (d) S2
According to the microhardness results of the layers in Fig.5, the samples prepared in aluminate electrolytes are harder than those prepared in silicon electrolytes. It is well known that the microhardness of the ceramic layer is decided by two factors: the phases in the layer and the density of the layer. The hard α-Al2O3 phase lays a good foundation for the microhardness of the layers prepared in four electrolytes. From the SEM cross-sectional micrographs, it is shown that the layers prepared in aluminate electrolytes are much denser than those prepared in silicate electrolytes, which results in the microhardness advantage in aluminate electrolytes. It is found that the addition of Na2SiF6 can significantly increase the layer microhardness by enhancing the layer density. Based on the generation of α-Al2O3 and the densification effects of Na2SiF6, the microhardness of the layers in A2 and S2 process (added with Na2SiF6) has a more significant improvement than the layers in A1 and S1 (without Na2SiF6), respectively.

Fig.5 Microhardness of ceramic layers prepared in four kinds of electrolytes
4 Conclusions
The four PEO processes were separately carried out in four different kinds of alkaline silicate and aluminate electrolytes on 6061 Al substrate for 5 min under a hybrid voltage.
1) The surface morphologies of PEO layers in aluminate electrolytes present pancake structures, while the volcano structures are shown in the layer prepared in silicate electrolytes.
2) The main phases are γ-Al2O3 and α-Al2O3; furthermore, mullite particles are generated around the plasma discharged channels especially in silicate electrolytes.
3) It can be concluded that the addition of Na2SiF6 can accelerate the growth of the oxide layers and significantly increase the microhardness by enhancing the layer density.
References
[1] WirtZ G P, Brown S D, Kriven W M. Ceramic coatings by anodic spark deposition [J]. Material Manufacture Process, 1991, 6: 87-115.
[2] Xue W, Wang C, Deng Z, Chen R, Lai Y, Zhang T. Evaluation of the mechanical properties of microarc oxidation coatings and 2024 aluminium alloy substrate [J]. Journal of Physics: Condensed Matter, 2002, 14: 10947-10952.
[3] Curran J A, Clyne T W. Thermo-physical properties of plasma electrolytic oxide coatings on aluminium [J]. Surface and Coatings Technology, 2005, 199: 168-176.
[4] Wang Y, Lei T, Jiang B, Guo L. Growth, microstructure and mechanical properties of microarc oxidation coatings on titanium alloy in phosphate-containing solution [J]. Applied Surface Science, 2004, 233: 258-267.
[5] Gu W C, Lv G H, Chen H, Chen G L, Feng W R, Yang S Z. Characterisation of ceramic coatings produced by plasma electrolytic oxidation of aluminum alloy [J]. Mat Sci Eng A, 2007, 447: 158- 162.
[6] Yeronkhin A L, Nie X, Leyland A, Matthews A, Dowey S J. Plasma electrolysis for surface engineering [J]. Surface and Coatings Technology, 1999, 122: 73-93.
[7] Barik R C, Wharton J A, Wood R J K, Stokes K R, Jones R L. Corrosion, erosion and erosion-corrosion performance of plasma electrolytic oxidation (PEO) deposited Al2O3 coatings [J]. Surface and Coatings Technology, 2005, 199: 158-167.
[8] Sun X T, Jiang Z H, Xin S G, Yao Z P. Composition and mechanical properties of hard ceramic coating containing α-Al2O3 produced by microarc oxidation on Ti-6Al-4V alloy [J]. Thin Solid Films, 2005, 471: 194-199.
[9] WEI T B, YAN F Y, TIAN J. Characterization and wear- and corrosion-resistance of microarc oxidation ceramic coatings on aluminum alloy [J]. Journal of Alloys and Compounds, 2005, 389 (1/2): 169-176.
[10] WU H H, JIN Z S, LONG B Y. Characterization of microarc oxidation process on aluminum alloy [J]. Chinese Physics Letters, 2003, 20(10): 1815-1818.
[11] McPherson R. The enthalpy of formation of aluminium titanate [J]. Journal of Materials Science, 1973, 8: 851-858.
[12] Gnedenkov S V, Khrisanfova O A, Zavidnaya A G, Sinebrukhov S L, Kovryanov A N, Scorobogatova T M, Gordienko P S. Production of hard and heat-resistant coatings on aluminium using a plasma micro-discharge [J]. Surface and Coatings Technology, 2000, 123: 24-28.
Foundation item: Korea Research Foundation Grant (KRF-2006-005-J02703)
Corresponding author: Bon-Heuno KOO; Tel: +82-55-264-5431; E-mail: bhkoo@changwon.ac.kr
DOI: 10.1016/S1003-6326(08)60366-0
(Edited by YANG Bing)