
Coating of magnesium powder by rapid expansion of supercritical solution method
ZHANG Shu-hai(张树海)
School of Chemical and Environmental Engineering, North University of China, Taiyuan 030051, China
Received 10 April 2006; accepted 25 April 2006
Abstract: In order to solve the reserve stability problem of pyrotechnic explosives containing magnesium powder, a coating method named rapid expansion of supercritical fluid is studied. Continuous coating film of paraffin can be formed on the surface of magnesium powder when paraffin content is about 1.5%. Humidity resistance property of the coated magnesium is promoted obviously. The flame sensitivity of the coated magnesium is 4.8% lower than that of the original magnesium powder after mixed with oxidant. The burning velocity of the coated magnesium is 4.7% lower than that of the contrastive sample. This method may be used to enhance the reserve stability problem of pyrotechnic explosives and the safety of magnesium powder modifying process.
Key words: magnesium powder; coating; supercritical fluid; pyrotechnic explosive
1 Introduction
Powdered magnesium has a wide range of applications in various chemical, military, pharmaceutical, metallurgical, and agricultural industries. Specific applications include steel desulphurization, pyrotechnics, metal matrix composite fillers and powder metallurgy [1, 2]. Magnesium is a highly energetic material, i.e., it has a tendency to catch fire, especially in pyrotechnics industries.
Magnesium powder is one of the most important components of many pyrotechnic explosives because of its good burning and illuminating properties. However, magnesium is very active, and it also has a high affinity with oxygen and water. When it is affected with damp the following reaction will occur [2]:
Mg+2H2O == Mg(OH)2+H2↑+342 kJ (1)
Hydrogen gas can react with the oxygen agent of the pyrotechnic explosive. For Ba(NO3)2, an usual oxygen agent, the reaction is
Ba(NO3)2+8H2 == Ba(OH)2+2NH3+4H2O (2)
This reaction will accelerate if there are impurities such as Cu, Pb in magnesium. Chain reaction will take place because H2O is generated in the second reaction.
The first reaction is an exothermic reaction and hydrogen gas will be created. If stockpile condition is not appropriate, spontaneous combustion even explosion maybe occur. Hence surface of magnesium powder should be coated before blended with oxygen agent.
Familiar modification agents are wax and stearic acid. At present, there are two methods to coat magnesium powder.
In most circumstances, people want to coat the surface of magnesium by using some inert substances. Parrafin and stearic acid are usually used as the modifying agent. Melting-mixing and dissolving-mixing method had been used in the former. But the modifying effect is not very satisfactory and the coating agent on the surface is usually uneven. Because a great lot of organic solvent is always consumed, safety, enviro- nmental and agglomeration problems are inevitable.
PFEFFER and DAVE et al [1, 3, 4] invented a dry coating method at the beginning of this century. For this method, powder mixture is carried by an air stream generated by the blades rotating at very high speed and recirculates in the machine through the cycle tube. Coating is achieved due to the high impaction force and friction heat. Obviously, magnesium powder is very dangerous under the effect of high speed air current.
Aiming at the reserve stability problem of pyrotechnic explosives containing magnesium powder and the problem mentioned above, an environmentally friendly and economical coating process based on rapid expansion of supercritical solution technology (RESS) is studied.
2 Priciples of RESS coating method
Supercritical fluids (SFs) are fluids that are at temperatures and pressures above their critical points [5]. At critical points, SFs possess properties of both liquid and gas, with density values similar to those of liquids, and flow properties similar to those of gases. The density values of SFs enable substantial dissolving power, and the diffusivity of solutes is higher because of lower viscosity of solutes in SF, thus facilitating mass transfer. When the temperatures and pressures of SF solutions are lower than their critical points the solubility will drop sharply and the solute will be separated out. That is why we can use SF technology to coat the surface of powder[5-8].
A schematic diagram of the experimental apparatus is shown in Fig.1.
CO2 firstly passes through the filter where infinitesimal water in it is eliminated. It is heated and pressed to the supper critical state and flows to the dissolving chamber in which coating agent is dissolved at certain pressure and temperature. Then, the CO2-coating agent mixture will be carried to the nozzle through which it will be sprayed to the coating chamber and expanded to state near normal. The fluid current will fluidize the powder put aforehand in the coating chamber. In the meantime, coating agent will separate out because the solubility decreasing of CO2. Very fine particle of the coating agent will be generated because ultra high saturation is produced under the rapid expanding condition. Powder in the coating chamber can be coated by the coating agent if their surface properties are suited.
Maximum operating pressure and temperature of this equipment is 50 MPa and 100 ℃, respectively. The flux of inlet flow can be continuously adjusted from 0.25 L/min to 50 L/min. The nozzle is a stainless steel one with diameter of 100 μm and length/diameter of 200∶1.
3 Experimental
3.1 Materials
Magnesium powder used as the original materials is made by Northeast Light Alloy Co Ltd; it has a mean particle diameter of 200 μm. Food grade CO2 is used as the supercritical fluid medium. Paraffin with 64 mean molecular mass produced by Lanzhou Refinary Plant is used as coating agent.
3.2 Coating conditions
The experimental scale of magnesium powder is 5.0 g. The powder is coated under the conditions listed in Table 1.
Table 1 Experimental conditions for magnesium powder coating

3.3 Performances test
The coated magnesium powders are analyzed with S-530 scanning electron microscope.
Their moisture absorption performance is tested by the mass method used in pyrotechnics industry so as to validate the humidity resistance effect of coating. In the
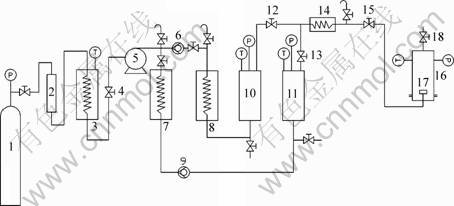
Fig.1 Schematic diagram of RESS coating method: 1 CO2 bottle; 2 Filter; 3 Refrigeration chamber; 4, 12, 13, 15, 18 Valve; 5 CO2 pump; 6, 9 Back valve; 7, 8, 14 Heat exchanger; 10 Dissolving chamber, 11 Spare chamber; 16 Coating chamber; 17 Nozzle
test both original and coated magnesium powders are exposed to 100% humidity at 65 ℃ for 48 h.
In order to verify the effect of the new method, burning performances of the original and coated magnesium powder are measured. Composites of the original magnesium powder and coated magnesium powder with potassium chlorate are prepared respectively according to zero oxygen balance rule as the tested samples.
Flame sensitivity of the mixture is measured by Bruceton method using fuse fire sensitivity instrument shown in Fig.2.
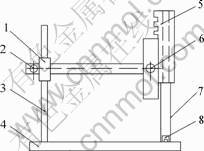
Fig.2 Flame sensitivity instrument: 1 Backstop; 2 Coarse adjusting handle; 3 Oriented column; 4 Foundation; 5 Fuse containing shaft; 6 Delicate adjusting handle; 7 Ruler; 8 Sample
Fuse of the test is common industrial product and is cut as sects with 7 cm length for use. Mixture sample used in each shot is 20 mg and put into a primer shell of 7.62 mm bullet. Bruceton method is adopted in the test to determine the 50% fire distance (H50).
In order to measure the burning velocity, an instrument is established. Its principle is shown in Fig.3. If the length between the two sensors and the alternation between the two sensors broken are known, the burning velocity can be calculated.
Sample density in the test is 1.2 g/cm3. Each sample is tested for 5 times and the average result can be drawn.
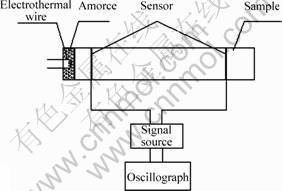
Fig.3 Schematic diagram of burning velocity test
4 Results and discussion
4.1 Surface morphology
From the photograph of SEM (Fig.4 to Fig.6), it can be seen that coating layers are formed on the surface of magnesium powder. When paraffin amount is about 1.5%, the coating layer is very uniform. As the former study[9], the mass of paraffin is also in direct ratio to the feeding time of CO2-paraffin solution. That is to say, the coating process can be controled accurately.
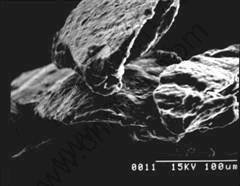
Fig.4 SEM photograph of coated magnesium with 1.52% paraffin
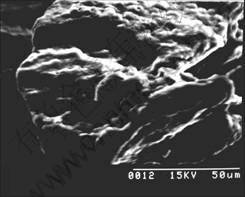
Fig.5 SEM photograph of coated magnesium with 3.03% paraffin
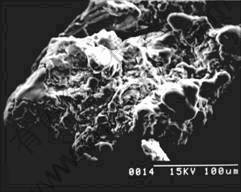
Fig.6 SEM photograph of coated magnesium with 5.81% paraffin
4.2 Humidity resistance of coated magnesium powder
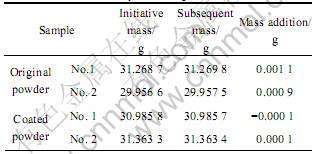
Moisture absorption testing results of coated magnesium containing 1.52% paraffin are listed in Table 2. It is indicated that magnesium powder coated with paraffin almost does not absorb moisture. This is because hydrophobic paraffin film is formed on the surface of magnesium. In the meanwhile, particles forming the film are very fine, and can even reach nanometer size [10, 11]. They can be embedded to the crack of magnesium, hence continuity of the coating film is better than that generated by other coating methods. Furthermore, superfine particles have lower surface energy of per unit area [12], which makes the film more difficult to be wetted and leads to more excellent humidity resistance properties.
Table 2 Moisture absorption testing results
4.3 Flame sensitivity
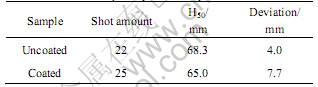
Flame sensitivities of composite made by coated magnesium powder (paraffin content 1.5%) powder and that made by original magnesium powder are tested. The results in Table 3 show that coating reduces the composite’s fire sensitivity, but the extent is only 4.8%. This indicate that coating by RESS process will not affect the igniting properties of pyrotechnics containing magnesium powder.
Table 3 Fire sensitivity compare of samples
4.4 Burning velocity
Burning velocity of both pyrotechnics containing original and coated magnesium powder respectively are tested and the results are listed in Table 4. From the results, it can be seen that the burning velocity of the pyrotechnics is only 4.7% lower after coated magnesium powder is used. That is to say, the effect of modification on the burning performances of magnesium powder is very slight.
Table 4 Burning velocity results

5 Conclusions
1) RESS technology can be used to magnesium powder coating, and continuous coating film of wax can be formed on the surface of magnesium powder.
2) No organic solvent is used and no mechanic frication comes into being in the RESS coating process, so it is a green and safe process.
3) Using RESS technology to coat magnesium powder can overcome the hygroscopic property but does not debase its burning properties evidently. Hence, this technology may be used to solve the reserve stability problem of pyrotechnic explosives and enhance the safety of magnesium powder modifying process.
References
[1] MUJUMDAR A, WEI D G, DAVE R N, et al. Improvement of humidity resistance of magnesium powder using dry particle coating [J]. Powder Technology. 2004, 140: 86-97.
[2] PAN Gong-pei, YANG Shuo. Pyrotechnics [M]. Beijing: Beijing Institute of Technology Press, 1996. 163-172. (in Chinese)
[3] WATANO S, PFEFFER R, DAVE R N, DUNPHY W. Dry particle coating by a newly developed rotating fluidized bed coater [A]. AIChE Symposium, Advanced Technology for Fluid-Particle System[C]. 1999, 321(95): 112-116.
[4] PFEFFER R, DAVE R N, WEI D G, RAMLAKHAN M. Synthesis of engineeredparticulates with tailored properties using dry particle coating[J]. Powder Technology, 2001, 117: 40-67.
[5] JUNG J, PERRUT M. Particle design using supercritical fluids: literature and patent survey [J]. Journal of Supercritical Fluids, 20, 2001, 20: 179-219.
[6] TSUTSUMI A, NAKAMOTO S, MINEO T, et al. A novel fluidized-bed coating of fine particles by rapid expansion of supercritical fluid solutions [J]. Powder Technology. 1995, 85: 275-280.
[7] SCHREIBER R, REINKE B, VOGT C, et al. High-pressure fluidized bed coating utilizing supercritical carbon dioxide[J]. Powder Technology, 2003, 138: 31-38.
[8] WANG Ting-jie, TSUTSUMI A, HASEGAWA H, et al. Mechanism of particle coating granulation with RESS process in a fluidized bed [J]. Powder Technology, 2001, 118: 229-235.
[9] ZHANG Shu-hai, GOU Rui-jun, ZHANG Jing-lin, et al. Study on the RESS coating technology of nitromine explosives [J]. Initiators & Pyrotechnics, 2004, (2): 20-23. (in Chinese)
[10] DEBENENDETTI P G. Homogenous nucleation in supercritical fluids [J]. AIChE Journal, 1990, 36(9): 1289-1298.
[11] YE Xiang-rong, WAI C M. Making Nanomaterials in supercritical fluids: A review [J]. Journal of Chemical Education,2003, 80(2): 198-204.
[12] ZHENG Rui-lun, LIANG Yi-pin. Influence of anharmonic vibration on surface energy of nanometer Ag particles[J]. Chinese Journal of Materials Research, 1997, 11(1): 37-41. (in Chinese)
(Edited by PENG Chao-qun)
Foundation item: Project (20031013) supported by Youth Science and Technology Foundation of Shanxi Province of China
Corresponding author: ZHANG Shu-hai; Tel: +86-351-3921446; E-mail: zsh93y@yahoo.com.cn