
Valence variation of arsenic in bioleaching process of
arsenic-bearing gold ore
CUI Ri-cheng(崔日成), YANG Hong-ying(杨洪英), CHEN Sen(陈 森), ZHANG Shuo(张 硕), LI Ke-feng(李科峰)
School of Materials Science and Metallurgy, Northeastern University, Shenyang 110004, China
Received 6 July 2009; accepted 25 December 2009
Abstract: The concentration and variational trend of As3+ and As5+, the bacterial resistance for the As3+ and As5+ and converting conditions from As3+ to As5+ were analyzed. The additive was used to prompt the bacterial leaching efficiency by changing valence state of arsenic. The results show that the concentration of As3+ is larger than that of As5+ in the lag phase. The concentration of As3+ decreases in the log phase, and is lower than that of As5+. HQ-0211 typed bacteria express better resistance for As3+ and As5+ and remain growing when the concentrations of As3+ and As5+ are above 6.0 g/L and 12.0 g/L, respectively. It is found that Fe3+ cannot oxidize As3+ singly as strong oxidant in the leaching system, but can cooperate with pyrite or chalcopyrite to do that. The oxidation of As3+ is prompted with addition of H2O2. The bacterial activity is improved in favor of bacterial leaching efficiency. NaClO restrains the bacterial growth to depress leaching efficiency because of the chloric compounds affecting bacterial activity.
Key words: As3+; As5+; bacterial leaching; arsenic resistance; oxidant; arsenic-bearing gold ore
1 Introduction
Arsenic-bearing gold ore is a common refractory gold ore. Arsenic of gold ore is very unfavorable to cyanide leaching[1-2]. Arsenic wraps gold grain in the form of arsenopyrite, which severely cuts off the cyanide from gold grain. Arsenic-bearing gold ore is easy to produce AsS33-, CNS-, S2O32-, AsO33- and AsO43- in cyanide solution. These products decrease the leaching efficiency because they exhaust cyanide and form compact film on the surface of gold grain to separate CN- and O2 from the grains in the leaching process. The oxidation pretreatment is used to depress or remove the arsenic in the gold ore in order that the gold is denuded before cyanide leaching[3-4]. Now pretreatment technologies for the ore are roasting oxidation, pressure oxidation and bacteria oxidation. Among them, the bacteria oxidation is famous for low cost, high efficiency and environmental protection[5-6]. Iron and sulfur in the ore are energy sources for bacteria to meet their metabolic demand. Then ore grade will be increased[7-8]. Refractory gold ore with high arsenic concentration has two impending problems to be solved, i.e. improving bacterial leaching efficiency and shortening oxidation period. Arsenic is the most influential compound on the bacterial activity in the leaching system and it is highly toxic substances. There are two types of As3+ and As5+ in the solution. The toxicity of As3+ is much higher than As5+[9-11]. Accelerating the transition from As3+ to As5+ is very important for decreasing the concentration of As3+, improving bacterial activity and increasing leaching efficiency. The present research of bacterial oxidation of arsenic-bearing gold ore is emphasized on the bacterial adsorption, bacterial extracellular polysaccharide and different single-mineral studies. In this work, a series of theoretical researches are emphasized on the valence state distribution of arsenic in the bacterial leaching process, bacterial resistance for As3+ and As5+, transitional conditions from As3+ to As5+ and oxidant for converting As3+ and depressing bacterial toxicity. The research provides important theoretical consults for more mature, fast, steady, economic and high-performance bacterial oxidation pretreatment of the high arsenic refractory gold ore.
2 Materials and experimental
2.1 Materials
Bacteria HQ-0211 used by test, which are mixed with leaching bacteria dominated by Thiobacillus ferrooxidans. They are screened and have been domesticated for a long time in the laboratory.
The medium is 9K that was composed of 3.00 g/L (NH4)2SO4, 0.10 g/L KCl, 0.50 g/L K2HPO4, 0.50 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2 and 44.30 g/L FeSO4·7H2O[12]. pH is 1.8 regulated by sulfuric acid.
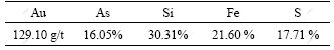
The ore sample comes from gold concentrate mine in Hunan Province, China, and the sample with size less than 0.038 mm is over 90%. The phase analysis shows that arsenic exists as arsenopyrite in the sample as shown in Fig.1. Table 1 lists the result of main elemental analysis.

Fig.1 XRD pattern of gold concentrate
Table1 Result of main element analysis
The chemicals used in the experiments included As2O3, Na2HAsO3·7H2O, H2O2, NaClO, NaOH, HCl, C6H5-CH3 and I2. All the chemicals were analytical reagents. All the aqueous solutions were prepared with the distilled water.
2.2 Experimental
2.2.1 Extractive separation of As3+ and As5+
The toluene was used to extract the As3+ from the inorganic phase to the organic phase quantificationally in the condition of strong acid. The As5+ remained in the organic phase. The As3+ was back-extracted to aqueous phase from the inorganic phase and titrated by iodine titer in the condition of weak base[13]. The As5+ was measured by hypophosphite titrimetric method[14].
2.2.2 Arsenic resistance of bacteria
10% of HQ-0211 typed bacteria with high activity were injected into the 200 mL of 9K medium with different concentrations of As3+ and As5+ and were shake-flask cultured in constant temperature shaking incubator at 44 ℃. The concentration gradient of As3+ was 0, 1.5, 3.0, 4.5 and 6.0 g/L. The concentration gradient of As5+ was 0, 3.0, 6.0, 9.0 and 12.0 g/L.
2.2.3 Bacterial leaching of gold concentrate ore
10% of HQ-0211 typed bacteria were injected into 500 mL of shake flask with 200 mL of 9K medium in constant temperature shake incubator at 44 ℃. After activation of bacteria, 10 g of gold concentrate ore was added into the pulp.
2.3 Analytical method
The electric potential, pH and concentration of Fe2+, TFe, As3+ and As5+ are determined in leach liquor in bioleaching and oxidant experiments per 24 h. Fe2+ and TFe are determined by dichromate method. The concentration of As3+ and As5+ are determined by toluene extraction-separation method.
The electric potential, pH and concentration of Fe2+ and TFe are determined in arsenic resistance experiment per 24 h. The bacterial reproduction is counted in blood counting chamber under the microscope. Growth curve is drawn.
3 Discussion
3.1 Concentration change of arsenic
Arsenic is the key negative factor in the biooxidation. The research of arsenic and its derivatives is the hotspot in the biohydrometallurgy. As3+ becomes the hotspots including its indigenous time, concentration change and transition to the arsenic in high valence because the toxicity of As3+ is stronger than that of As5+ [15-16]. The toluene extraction-separation method is used to pursue the concentration changes of As3+ and As5+ in the arsenic-bearing gold concentrate bioleaching process. Due to ferrous ion and sulfur element of ore as energy source for leaching bacteria, they obtained energy by oxidation them to grow, so the system redox potential and pH value changes can be indirectly reaction to the growth of bacteria and the disintegration of minerals. Bacteria adapted to the ore. Lag phase of bacteria only lasts three days. The fourth day is logarithm. At this time, arsenic-bearing ores are quickly oxidized and decomposed. Potential suddenly ascend,and pH value is declining. The fifth day and the sixth day are the stable phase. Due to ferrous ion and sulfur element are oxidized, no increase in potential. But bacteria are still in the production of acid phase, and pH value is declining as shown in Fig.2. The concentration of As3+ is higher than that of As5+ in the former three days, namely the lag phase. The concentration of As3+ reaches the climax, 4.63 g/L, in the 3rd day. The concentration of As3+ steps down until below As5+ in the log phase of bacteria as shown in Fig.3. The concentration of As3+ increases then decreases compared with the escalation of As5+ in bioleaching process. The change of arsenic phase is AsS2-→As3+→As5+ as shown in Fig.3. As3+ is produced and oxidized in bioleaching process. In the lag phase of bacteria, the indigenous rate is higher than the oxidized rate of the As3+ because of the weak activity of bacteria and oxidization. In the flushing log phase and stable phase of bacteria, escalating concentration of Fe3+ leads to the higher oxidizing rate than indigenous rate. The As5+ has higher concentration than As3+. In other words, the activity of bacteria increases with the decrease of As3+ concentration and increase of As5+ concentration. Thus, transition from As3+ to As5+ has great influence on the biooxidation pretreatment. A large number of arsenopyrite, pyrite and other sulfide minerals are oxidized in bioleaching process. At the end of the experiment, the mass loss rate is 61.8% and the dearsenization rate is 98.2%.
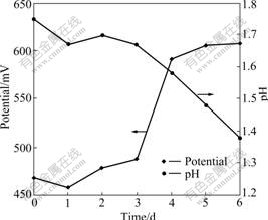
Fig.2 Potential and pH changes with bioleaching time
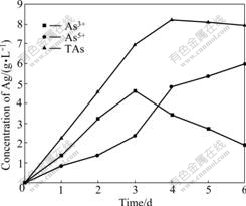
Fig.3 Concentration changes of As3+, As5+ and TAs with bioleaching time
3.2 As3+ and As5+ resistances of bacteria
Because As3+ and As5+ coexist in the bioleaching system, As3+ and As5+ resistances of bacteria were studied further. This work will help with formulations of pulp density and ore blending in bioleaching process. The results of experiment show that the bacterial growth rate and the activity decrease with increasing concentrations of As3+ and As5+. Bacterial activity is seriously affected with the gradual increase in the concentration of As3+. Ferrous ion as energy cannot be oxidized, resulting in potential increased slowly. When the concentration of As3+ is 6.0 g/L, the bioleaching time of bacterial lag phase is 264 h. No restrain happens when the concentration of As3+ is below 1.5 g/L, as shown in Fig.4 and Fig.5. As5+ also has a great impact on the bacteria, but its toxicity is weaker than As3+. When the concentration of As5+ is 12.0 g/L, the bioleaching time of bacterial lag phase is 264 h. No restrain happens when the concentration of As5+ is below 3.0 g/L, as shown in Fig.6 and Fig.7. The lag phase is extended with the increase of arsenic concentration because the bacteria need time to adjust metabolism and catalyze its enzyme, coenzyme and intermediate to adapt to new environment. In this phase, the bacterial growth is slow and the oxidizing efficiency is low for ferrous ion. The bacteria may meet the high arsenic gold concentrate ore with 16.05% arsenic in the section 3.1 because the resistances of As3+ and As5+ are above 6.0 g/L and 12.0 g/L as shown in Figs.3, 5 and 7. Toxicity of As3+ is 60 times higher than that of As5+ for the human body and animal body[17-19]. But the result of this work is 2 times for the bacterial leaching activity experiments as shown in Fig.5 and Fig.7. Thus, the further research is needed for the toxicity theory of arsenic ion for the leaching bacteria.
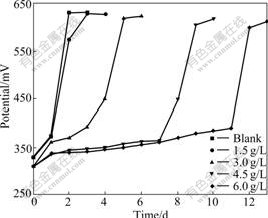
Fig.4 Potential change with bioleaching time at different As3+ concentrations

Fig.5 Bacteria count change with bioleaching time at different As3+ concentrations
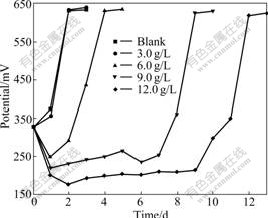
Fig.6 Potential change with bioleaching time at different As5+ concentrations
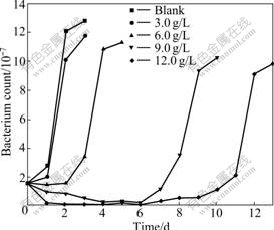
Fig.7 Bacteria count change with bioleaching time at different As5+ concentrations
3.3 Analysis of converting conditions from As3+ to As5+
The bacterial activity steps up from As3+ to As5+ in the process of biooxidation. Thus, it is important to study the converting conditions from As3+ to As5+ in the bacterial leaching process. The direct oxidation and indirect oxidation of the main body are bacteria and Fe3+ in bacterial oxidation system, therefore, to analyze As3+ to As5+ conversion conditions respectively, the bacteria, the medium of Fe3+ and pyrite often associated with gold ore were studied. The activated bacterial liquid is placed into four shake-flasks of 500 mL with 200 mL of 2 g/L As3+ standard liquid, as listed in Table 2.
Table 2 Different conditions of oxidation test for As3+
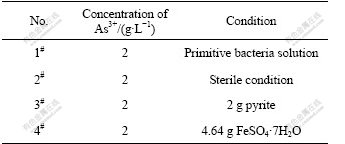
The converting of As3+ in the bacteria system is compared with asepsis after the bacteria are filtrated in the 2# flask through microporous membrane. The conversion ratios are zero in the 1# and 2# flasks after three days germiculture. This shows that the leaching bacteria cannot convert As3+ to higher valence state in the simplex 9K culture medium and Fe3+ does not play its role as oxidant, as listed in Table 3. 2 g pyrite with Fe3+ as strong oxidant added into the 3# flask is compared with 4# flask with 4.64 g FeSO4·7H2O. This is the same amount of iron in 2 g pyrite. The conversion ratio of As3+ reaches 35.61% and progressively rises in the 3# flask after being cultured for three days, on the contrast, none in the 4# flask, as listed in Table 3. 2 g FeSO4·7H2O is added into the 4# flask to ensure the mole ratio of iron to arsenic to convert As3+ to As5+ in the solution. After one day’s culture, As3+ isn’t oxidized in the ratio of 10:1 for iron to arsenic, and 480 mV in the 3# flask, which proves high concentration of Fe3+ and high electric potential are not preconditions to convert As3+ to As5+ in the leaching system. A part of As3+ is also oxidized after addition of chalcopyrite. O2 in the air is an electron acceptor in addition to Fe3+ in leaching system. To analyze the electron acceptor in the process of As3+ →As5+, biooxidation of pyrite is analyzed. XPS shows that O2- exists on the surface of pyrite when the flaky pyrite is oxidized by leaching bacteria as aerobic from low valence state to high valence state in the process of biooxidation. When the environment changes, EPS (extracellular polysaccharide) is secreted in the outer layer to promote oxidation of As3+. 3% EDTA is used to extract the EPS in the 3# and 4# flasks. The results show that the EPS concentration in the 3# flask is two times higher than that in the 4# flask because the secretion is stimulated by the addition of pyrite. Fig.8 shows that the bacteria are adhered to the surface of ore by the sticky EPS which controls the transference and exchange between the bacterial surface and environment. SAND et al[20] found that Fe3+ concentration in the EPS is much higher than that in the solution which prompts the oxidation of elements in low valence state in the system, and oxidoreductase secreted is congregated in the EPS to accelerate transference of electron in the bacterial enzyme system and to cause redox reaction to process easily. As exothermic reaction, the thermal energy relaxed by bacterial leaching catalyzes the reaction.
Table 3 Oxidation ratio of As3+ after being cultured for different time (%)


Fig.8 SEM image of bacteria adhering to surface of ore
3.4 Influence on bioleaching process by oxidant
The arsenic is notorious for toxicity and danger, and the oxidant is applied widely in the arsenic-bearing industrial wastewater. The section 3.1 shows that the trivalent arsenic exists in the former half part of the bioleaching process. The addition of oxidant oxidizes As3+ rapidly to depress the toxicity of arsenic and shorten the lag phase to enhance bacterial activity. It is very important to deal with long bioleaching period. It is easy to remove As5+ through coprecipitation, flocculation and absorption in the post processing. The oxidant has two sides because it promotes the oxidation of trivalent arsenic and restrains the bacterial growth. 30% of 0.3 mL H2O2 and 10% of 0.5 mL NaClO are added into at the 48th hour with high concentration of As3+. H2O2 leads to the lower concentration of As3+ than the blank sample from the 3rd day to the end of bacterial leaching, as shown in Fig.9. The reduced As3+ leads to the enhanced bacterial activity in the shake-flask with H2O2 and large leaching oxidizing rate. The total arsenic concentration exceeds 0.39 g/L which is larger than that of the blank sample and trends to stabilize in the 3rd day after reaching maximum dearsenization rate, as shown in Fig.10. The concentration of As3+ in the shake-flask with NaClO is lower than that of the blank sample, but the total arsenic concentration in the shake-flask with NaClO is also lower than that of the blank sample, as shown in Fig.10. The results show that bacteria are unfit for the environment and the activity and the leaching efficiency decreases. The As3+ liberating from the ore leads to the lower concentration of As3+ than the blank sample. The observation through the microscope shows that the growth rate and activity are lower than those in the other two shake-flasks. H2O2 remains more stable in the acid environment than the alkaline, but lots of ferric ion, sulfur ion and arsenic ion catalyze the decomposition rapidly in the solution at 44 ℃. The purpose is to depress the toxicity of arsenic for the bacteria and to improve the bacterial activity because the oxidation of H2O2 disappears in a short time and does not restrain the bacteria. NaClO is decomposed to HClO, Cl2 and Cl-
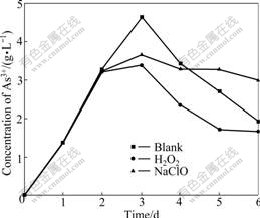
Fig.9 Influence of leaching time on concentration change for As3+ by H2O2 and NaClO
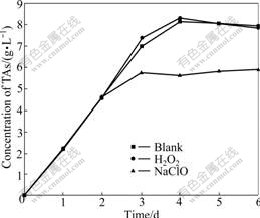
Fig.10 Influence of leaching time on total arsenic concentration change by H2O2 and NaClO
rapidly because of its instability and catalysis of other ions. The chloric compounds restrain bacterial activity strongly, which leads to low bacterial oxidizing rate and dearsenization rate.
4 Conclusions
1) The bacterial activity increases with the decrease of As3+ concentration and increase of As5+ concentration in the bioleaching process. In the lag phase, the concentration of As3+ is higher than that of As5+, contrarily it is lower in the log phase and stable phase.
2) The bacteria HQ-0211 have perfect resistances for As3+ and As5+. When the concentration of As3+ and As5+ are above 6.0 g/L and 12.0 g/L, the bacteria remain growth. Stable phase of the bacterial concentration may reach 1.0×108 cell/mL or more.
3) Fe3+ cannot convert As3+ to As5+ as strong oxidant in the simplex leaching system, but can cooperate with pyrite and chalcopyrite.
4) H2O2 has limited oxidation for As3+ and promotes the bacterial oxidizing leaching process. NaClO in the leaching system is unstable, and HClO, Cl2, Cl-and other chlorinated substances of decomposition products affect bacterial activity, leading to descend of leaching efficiency.
References
[1] SCHMITZ P A, DUYVESTEYN S, JOHNSON W P. Adsorption of aurocyanide complexes onto carbonaceous matter from pregrobbing Goldstrike ore [J]. Hydrometallurgy, 2001, 61: 121-135.
[2] TONG Lin-lin, JIANG Mao-fa, YANG Hong-ying, YIN Shu-yan. Experimental investigation on gold extraction from high-As concentrate in Hunan province through bio-oxidation/cyanadation [J]. Precious Metal, 2008, 29(1): 15-18. (in Chinese)
[3] YAHYA A, JOHNSON D B. Bioleaching of pyrite at low pH and low redox potentials by novel mesophilic gram-positive bacteria [J]. Hydrometallurgy, 2002, 63(2): 181-188.
[4] ZI Jian-wei, YANG Hong-ying, GONG En-pu, YANG Li, ZHAO Yu-shan, FAN You-jing. A review on biooxidation pretreatment of arsenric-bearing refractory gold ores [J]. Precious Metal, 2005, 26(1): 66-70. (in Chinese)
[5] AKCIL A. Potential bioleaching developments towards commercial reality Turkishmetalmining’s future [J]. Minerals Engineering, 2004, 17(3): 477-480.
[6] WATLINGH R. The bioleaching of sulphide mineralswith emphasis on coppersulphides—A review [J]. Hydrometallurgy, 2006, 84(1/2): 81-108.
[7] EHRILICH H L. Past, present and future of biohydrometallurgy [J]. Hydrometallurgy, 2001, 59(2/3): 127-134.
[8] SAMPSON M I, PHILIPS C V, BLAKE R C. Influence of the attachment of acidophilic bacteria during the oxidation of mineral sulfides [J]. Minerals Engineering, 2000, 13(4): 373-389.
[9] WANG Bin. Development of inorganic arsenic content analysis and critical number in food [J]. Journal of Public Health and Preventive Medicine, 2006, 17(2): 48-49. (in Chinese)
[10] KANG Jia-qi, JIN Yin-long. Study progress of adverse effects of arsenic on health [J]. Journal of Hygiene Research, 2004, 33(3): 372-375. (in Chinese)
[11] APOSHIAN H V, ZAKHARYAN R A, AVRAM M D, SAMPAYO R A, WOLLENBERG M L. A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species [J]. Toxicol Appl Pharmacol, 2004, 198: 327-335.
[12] CHARYA C A, KAR R N, SUKLA L B. Bacterial removal of sulphur from three different coals [J]. Fuel, 2001, 80: 2207-2216.
[13] HUANG Bao-gui. Investigation on the method of extraction-separation for high contents of As(Ⅲ) and As(V) [J]. Metallurgical Analysis, 1995, 15(4): 7-10. (in Chinese)
[14] SUN Shu-yuan, SUN Ling-gao, YIN Qi-xi, FU Bin. The mineral and nonferrous metals analysis manual [M]. Beijing: Metallurgy Industry Press, 1990: 112-113. (in Chinese)
[15] HE Qiu-hong, ZENG Xi-bo. Form transformation of arsenic in soil and corresponding analyzing methods [J]. Chinese Journal of Applied Ecology, 2008, 19(12): 2763-2768. (in Chinese)
[16] SHI Jia-wei, CHEN Xiao-hong. Survey on the inorganic arsenic and its valence in food [J]. China Preventive Medicine, 2005, 6(3): 206-208. (in Chinese)
[17] BAI Ai-mei, LI Yue, FAN Zhong-xue. Harmness on the human body by arsenic [J]. Studies of Trace Elements and Health, 2007, 24(1): 61-62. (in Chinese)
[18] ZUO Jin-long, CUI Fu-yi. A review of studies on pollutants and treatment techniques development in water supply [J]. Carcinogenesis Teratogenesis & Mutagenesis, 2007, 19(3): 174-180. (in Chinese)
[19] HU Liu-jie, BAI Ling-yu, LI Lian-fang, ZENG Xi-bai. The current research and trend on the speciation and bioavailability of arsenic in soils [J]. Journal of Nuclear Agricultural Sciences, 2008, 22(3): 383-388. (in Chinese)
[20] SAND W, GEHRKE T, JOZSA P G, SCHIPPER A. Direct versus indirect bioleaching [J]. Process Metallurgy, 1999, 9(1): 27-49
Foundation item: Projects(50674029, 50874030) supported by the National Natural Science Foundation of China; Project(2006AA06Z127) supported by the National High-tech Research and Development Program of China; Project(20060145015) supported by Specialized Research Fund for the Doctoral Program of Higher Education, China
Corresponding author: YANG Hong-ying; Tel: +86-24-83680373; E-mail: yanghy@smm.neu.eu.cn
DOI: 10.1016/S1003-6326(09)60274-0
(Edited by LI Xiang-qun)