
In vitro biocompatibility of titanium-nickel alloy with titanium oxide film by H2O2 oxidation
HU Tao(胡 涛)1, CHU Cheng-lin(储成林)1, YIN Li-hong(尹立红)2,
PU Yao-pu(浦跃朴)2, DONG Yin-sheng(董寅生)1, GUO Chao(郭 超)1,
SHENG Xiao-bo(盛晓波)1, CHUNG Jonathan-CY(钟志源)3, CHU Paul-K(朱剑豪)3
1. School of Materials Science and Engineering, Southeast University, Nanjing 211189, China;
2. School of Public Health, Southeast University, Nanjing 210096, China;
3. Department of Physics and Materials Science, City University of Hong Kong, Hong Kong, China
Received 28 October 2006; accepted 17 January 2007
Abstract: Titanium oxide film with a graded interface to NiTi matrix was synthesized in situ on NiTi shape memory alloy(SMA) by oxidation in H2O2 solution. In vitro studies including contact angle measurement, hemolysis, MTT cytotoxicity and cell morphology tests were employed to investigate the biocompatibility of the H2O2-oxidized NiTi SMAs with this titanium oxide film. The results reveal that wettability, blood compatibility and fibroblasts compatibility of NiTi SMA are improved by the coating of titanium oxide film through H2O2 oxidation treatment.
Key words: NiTi shape memory alloy; titanium oxide film; biocompatibility; wettability; cytocompatibility
1 Introduction
Nickel-titanium alloy (NiTi) is a metallic biomaterial known for its unique properties such as shape memory effect, super-elasticity and corrosion resistance. It is a promising material for surgical implants in orthopedics[1-2]. However, the large amount of nickel element in NiTi alloy is considered to be of great concern. For long term implantation, the nickel ions can be leached from the bulk and can induce hypersensitive reactions and tissue necrosis[3].
Since the leaching out of nickel ions may cause toxic reactions between implant and tissues inside the human body, we aim at producing barrier layer to impede the out-leaching of nickel ions. Titanium oxide coatings on NiTi shape memory alloy(SMA) that can be obtained by many methods are thought to be a useful way to suppress nickel ions out-leaching[4-7]. WU et al[8] found that titania film can be prepared on titanium by oxidation in H2O2 solution. Further studies showed that both in vitro and in vivo biocompatibility of titanium were improved[9-11]. In our previous study, we have successfully prepared the titania film on NiTi SMA with H2O2 oxidation treatment[12]. The aim of present investigation is to characterize in vitro biocompatibility of the H2O2-oxidized NiTi SMA with this titanium oxide film.
2 Experimental
2.1 Sample preparation and surface analysis
A commercially available NiTi (50.8% Ni, molar fraction) SMA plate for medical applications was cut into small rectangular blocks (10 mm×10 mm×1 mm). All samples were ultrasonically cleaned in acetone and deionized water for 10 min respectively, and then chemically polished to remove native surface oxides for 10 min in Kroll’s reagent (a mixture of 2 mL hydrofluoric acid (HF, 40%), 4 mL nitric acid (HNO3, 66%) and 994 mL deionized water). The samples were subsequently ultrasonically washed in acetone for 10 min and in deionized water for 10 min. They were divided into five groups. The first group was used as control (denoted as chemically-polished samples). The second and the third one were further oxidized in the aqueous solutions containing 10% H2O2 at 60 ℃ and 100 ℃, respectively. The last two groups were oxidized in aqueous solutions containing 30% H2O2 at 60 ℃ and 100 ℃, respectively. Each group consisted of samples oxidized for 1, 2, 5, 12 and 24 h, respectively. All oxidized samples were ultrasonically rinsed with deionized water for 10 min (denoted as 10% H2O2- oxidized samples and 30% H2O2-oxidized samples, respectively).
In order to remove surface contamination by carbon-containing or water molecules absorbed from the environment, a surface layer about 10 nm in thickness of the sample was firstly cleaned by sputtering with Ar+ ion beam. Then the depth profiles were determined by X-ray photoelectron spectroscopy using a VG Scientific ESCALAB 5 spectrometer with monochromatic Al Kα (1 486.6 eV) X-ray radiation. The thickness of oxide film on the sample was estimated based on sputtering rates calculated from a SiO2 reference under similar conditions.
2.2 In vitro studies
Contact angles were measured using liquid drop method by a face contact angle goniometer (JC2000B, China). A 10 μL droplet of deionized water was dripped on the sample surface. Then the contact angle between the drop and the substrate was measured.
In hemolysis test, 8 mL blood was freshly collected from a rabbit and then diluted with 10 mL 0.9% saline. Each sample was put into a test tube with 10 mL saline and incubated at 37 ℃ for 30 min. Then 0.2 mL diluted blood was added into each test tube and the incubation continued for another 60 min. After incubation, the suspension was centrifuged at 2 500 r/min for 5 min. The absorbance of the supernatant fluid was measured by a spectrophotometer (UV240, China). The positive control was blood/deionized water mixture and the negative control was blood/saline mixture. The hemolysis ratio (RH) was calculated according to the following formula:

where Dt, Dn and Dp are the absorbances of the treatment group, the negative control group and the positive control group, respectively.
Fibroblasts L929 were used to investigate the cytocompatibility of the H2O2-oxidized and chemically- polished NiTi samples. The cells were cultured in Dulbecco’s minimum essential media(DMEM) supplemented with 15% (volume fraction) fetal calf serum (FCS, Invitrogen) at 37 ℃ in an atmosphere of 5% CO2 and 95% air. The ultraviolet cleaned NiTi samples were fixed onto the bottom of a 24-well tissue culture plate. 1 mL cell suspension consisting of 1×105 cells was then seeded onto the surface of the H2O2-oxidized, chemically-polished NiTi samples and wells without any NiTi plates serving as negative control for the MTT (3-[4,5-dimethy-lthiazol-2-yl]-2, 5-diphenyltetrazoliumbromide) cytotoxicity test. The 24-well plates were then incubated at 37 ℃ in an atmosphere of 5% CO2 and 95% air.
After 24 h culture, the medium in all the wells was replaced with 100 μL 0.5% MTT and 400 μL DMEM and the samples were incubated at 37 ℃ in an atmosphere of 5% CO2 and 95% air for 4 h. Then the cells were dissolved by dimethylsiloxane(DMSO) and agitated with a plate shaker for 10 min. The optical absorbance of the fluid was read at a wavelength of 575 nm (reference 620 nm). The relative growth rate (Rrg) of fibroblasts on the surface of H2O2-oxidized and chemically polished NiTi samples was calculated according to the following formula:

where Dt, Dn, At and An are the absorbances of the treatment group and the negative control group, the area of NiTi sample and the well of 24-well tissue culture plate for negative control, respectively. All results were expressed as mean±standard deviation(SD) from the 3 identical samples at each time point in each test. Differences were considered statistically significant at p<0.05.
In the cell morphology test, the method initially was similar to the protocol for the MTT test. The samples were treated in the same manner up to the incubation. After 1-2 days, the morphology of fibroblasts L929 in the vicinity of NiTi samples was microscopically photographed by an inverted microscope (IX70, Olympus, Japan) with a digital camera (DSC-P72, SONY).
3 Results
Titania film could be formed on NiTi SMA after oxidized in H2O2 solution as reported in Ref.[12]. Fig.1 shows the XPS depth profiles obtained from the chemically polished NiTi sample and H2O2-oxidized one. The nickel concentration near the surface of oxidized NiTi is much lower compared with that of the chemically polished one. Thus the surface nickel concentration is highly suppressed by oxidation with H2O2 solution. The thickness of titanium oxide film on the surface of NiTi samples oxidized with the boiling 30% H2O2 solution for 2 h is estimated in the described manner as 0.5 μm. The inconsistency of the steady-state values of Ni and Ti in the sample after the sputtering of Ar+ ion beam with the chemical compositions of the untreated NiTi SMA (50.8% Ni, molar fraction) may be due to preferential sputtering of Ti[6].
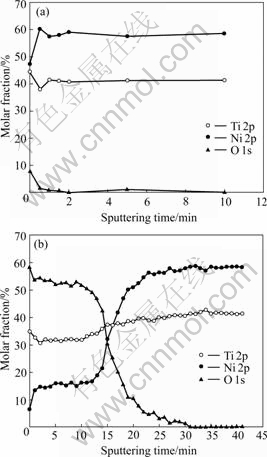
Fig.1 XPS depth profiles of surfaces of NiTi SMAs: (a) Chemically-polished NiTi; (b) 30% H2O2-oxidized NiTi
Figs.2 and 3 show the changes of water contact angles of the oxidized NiTi SMA surfaces with the oxidation time in H2O2 solutions. The contact angles decrease with increasing of the oxidation time for all types of samples. After 24 h oxidation, the contact angles of NiTi SMAs oxidized by 30% H2O2 solution at 60 ℃ and 10% H2O2 solution at 100 ℃ decrease to 21.9? and 28.8?, respectively, which are much lower than 65.5? of the chemically-polished one. It can be concluded that the wettability of NiTi can be improved by the H2O2 oxidation.

Fig.2 Changes of water contact angles of oxidized NiTi SMA surfaces with oxidation time in 10% H2O2 solution at 60℃ (a) and 100℃ (b)

Fig.3 Changes of water contact angles of oxidized NiTi SMA surfaces with oxidation time in 30% H2O2 solution 100 ℃ (a) and 60 ℃ (b)
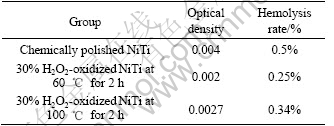
Table 1 lists the results of hemolysis tests of the chemically polished sample and those oxidized for 2 h. Hemolytic activity was assessed by determining hemoglobin release under static conditions. The hemolysis rates of the H2O2-oxidized NiTi SMA both at 60 ℃ and 100 ℃ and the chemically-polished one are less than 5%, which indicates all of them meet the requirements of biomaterials for implantation. However, the hemolysis rate of the oxidized NiTi sample in 30% H2O2 solution at 60 ℃ is less than that of the oxidized one in 30% H2O2 solution at 100 ℃. A higher hemolysis rate (0.5%) means a higher extent of hemolysis occurred on the surface of the chemically polished NiTi SMA. Therefore, the blood compatibility of NiTi SMA can also be improved by surface oxidation in H2O2 solution.
Table 1 Results of hemolysis test
MTT reagent is a pale yellow substance that is reduced to a dark blue formazan product when incubating with viable cells. Therefore, the production of formazan can reflect the level of cell viability. Table 2 lists the difference in the cytotoxicity of the 30% H2O2-oxidized samples for 2 h at 60 ℃ and 100 ℃. It can be found that the optical density(OD) value and relative growth rate(RGR) of the NiTi sample oxidized at 60 ℃ are higher than those of the one oxidized at 100 ℃. The OD value and RGR of both 30% H2O2-oxidized samples are much higher than those of the chemically- polished one. The higher growth rate of fibroblasts confirms that the titanium oxide film without cytotoxic effect on the H2O2-oxidized NiTi SMA can promote the proliferation of fibroblasts.
Table 2 Results of MTT test

Cell adhesion, spreading and migration on substrates are the first sequential reactions when coming into contact with a material surface, which is crucial to cell survival. The cellular behavior on biomaterial is an important factor for evaluation of its biocompatibility. Fig.4 shows the fibroblasts morphology in the vicinity of 30% H2O2-oxidized NiTi SMA after culturing. It is clearly found that the fibroblasts display a spindle-like feature that is the normal morphology after 48 h co-culture, and there is a good cell adhesion to the H2O2-oxidized SMA surface.

Fig.4 Microscopic views on vertical site of 30% H2O2-oxidized NiTi SMA after co-culture with fibroblasts L929 for 48 h (Dark parts in images are NiTi plates): (a) Oxidized at 60 ℃ for 2 h; (b) Oxidized at 100 ℃ for 2 h
4 Discussion
As indicated by the depth profiles of Fig.1(b), the surface nickel content of NiTi SMA oxidized in H2O2 solutions is suppressed by the formation of titanium oxide film, and the graded interface may enhance the adhesion of titanium oxide film to NiTi matrix. It is also found that titanium oxide films improve the surface wettability of NiTi sample. LAWRENCE et al[13] reported that the increasing of surface oxygen content can result in the improvement of wettability on the surface of titanium alloy (Ti6Al4V). As shown in Figs.2 and 3, the surface contact angles decrease with increasing of the oxidation time for all oxidized NiTi SMAs, which may be attributed to the increasing of surface oxygen content and more titanium oxide formed on NiTi SMAs by longer time oxidation.
The interaction process between blood and biomaterial is very complicated, and it is not yet clear in every detail. However, many researches have proved that titanium oxides have good blood compatibility[14]. In this work, the blood compatibility of the H2O2-oxidized SMAs has been improved compared with that of the chemically-polished one, which also suggests that titanium oxides have better blood compatibility than that of NiTi matrix.
The release of Ni atoms from the surface of NiTi SMA into the boiling water was found by SHABALOVSKAYA and ANDEREGG[15]. WEVER et al[16] also showed that a parabolic law for nickel ions released with an initial release rate of 14.5×10-7 μg/cm2, and then decreased rapidly due to the build-up of TiO2 passive layer. ARMITAGE et al[17] reported that heat-treated NiTi alloy possessed good biocompatibility but the thrombogenicity was significantly reduced because of the formation of titania and other titanium oxides on its surface. We propose the use of H2O2 oxidation treatment to improve the surface properties of NiTi alloy in order to achieve better corrosion resistance and reduce nickel out-leaching. Our results of MTT cytotoxicity and fibroblasts morphology tests indicate that the H2O2 oxidized NiTi samples show better cell proliferation and adhesion to NiTi surface, which may correspond to its improved wettability. In general, wettability is considered as one of the important surface properties that can affect the cellular behaviors, i.e., adhesion, morphologic change and proliferation greatly[18]. TAKEBE et al[19] reported that the improved wettability could cause a higher level of cell adhesion and proliferation on the surface of biomaterial. In addition, the improvement of cell compatibility may also be due to the suppression of nickel leaching from the NiTi alloys with the presence of titanium oxide layer by H2O2 oxidation. The formation of titanium oxide on NiTi SMA sharply decreases the surface Ni concentration. It is logical to believe that the low nickel concentration near the surface reduces the risk of its leaching, thus the biocompatibility of NiTi alloy is improved.
5 Conclusions
1) Using H2O2 oxidation treatment, a titanium oxide layer with a graded interface to NiTi matrix was fabricated. The amount of nickel near the surface of NiTi shape memory alloy is thus suppressed.
2) The wettability is improved markedly through surface oxidation in H2O2 solutions, especially for the oxidized NiTi SMA with a longer oxidation time in 30% H2O2 solution. The blood compatibility of NiTi SMA is enhanced by H2O2 oxidation.
3) MTT and cell morphology tests indicate that the oxidized NiTi surfaces are cytologically compatible allowing the attachment and proliferation of fibroblasts due to the formation of titanium oxide film.
References
[1] YEUNG K W K, POON R W Y, LIU X Y, HO J P Y, CHUNG C Y, CHU P K, LU W W, CHAN D, CHEUNG K M C. Investigation of nickel suppression and cytocompatibility of surface-treated nickel-titanium shape memory alloys by using plasma immersion ion implantation [J]. J Biomed Mater Res, 2005, A72(3): 238-245.
[2] SHABALOVSKAYA S A. Surface, corrosion and biocompatibility aspects of nitinol as an implant material [J]. Biomed Mater and Eng, 2002, 12(1): 69-109.
[3] SHEVCHENKO N, PHAM M T, MAITZ M F. Studies of surface modified NiTi alloy [J]. App Surf Sci, 2004, 235(1/2): 126-131.
[4] CHU P K. Bioactivity of plasma implanted biomaterials [J]. Nuclear Instruments and Methods in Phys Res, 2006, B242(1/2): 1-7.
[5] LIU J X, YANG D Z, SHI F, CAI Y J. Sol-gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement [J]. Thin Solid Films, 2003, 429(1/2): 225-230.
[6] FIRSTOV G S, VITCHEV R G, KUMAR H, BLANPAIN B, HUMBEECK J V. Surface oxidation of NiTi shape memory alloy [J]. Biomaterials, 2002, 23(24): 4863-4871.
[7] CHENG F T, SHI P, MAN H C. Nature of oxide layer formed on NiTi by anodic oxidation in methanol [J]. Mater Lett, 2005, 59(12): 1516-1520.
[8] WU J M, HAYAKAMA S, TSURU K, OSAKA A. Porous titania films prepared from interactions of titanium with hydrogen peroxide solution [J]. Scripta Mater, 2002, 46(1): 101-106.
[9] TAKEMOTO S, YAMAMOTO T, TSURU K, HAYAKAMA S, OSAKA A, TAKASHIMA S. Platelet adhesion on titanium oxide gels: Effect of surface oxidation [J]. Biomaterials, 2004, 25(17): 3485-3492.
[10] KANEKO S, TSURU K, HAYAKAMA S, TAKEMOTO S, OHTSUKI C, OZAKI T, INOUE H, OSAKA A. In vivo evaluation of bone-bonding of titanium metal chemically treated with a hydrogen peroxide solution containing tantalum chloride [J]. Biomaterials, 2001, 22(9): 875-881.
[11] WANG X X, HAYAKAM S, TSURU K, OSAKA A. Improvement of bioactivity of H2O2/TaCl5-treated titanium after subsequent heat treatments [J]. J Biomed Mater Res, 2000, 52(1): 171-176.
[12] CHU C L, ZHOU J, CHUNG C Y, PU Y P, LIN P H. In situ formation of titania film on NiTi alloy treated with hydrogen peroxide solution at low temperature [J]. Trans Nonferrous Met Soc China, 2005, 15(4): 834-838.
[13] LAWRENCE J, HAO L, CHEW H R. On the correlation between Nd:YAG laser-induced wettability characteristics modification and osteoblast cell bioactivity on a titanium alloy [J]. Surf Coat Technol, 2006, 200(18/19): 5581-5589.
[14] HUANG N, YANG P, CHANG X, LENG Y X, ZHENG X L, CAI G J, ZHEN Z H, ZHANG F, CHEN Y R, LIU X H, XI T F. Blood compatibility of amorphous titanium oxide films synthesized by ion beam enhanced deposition [J]. Biomaterials, 1998, 19(7/9): 771-776.
[15] SHABALOVSKAYA S A, ANDEREGG J W. Surface spectroscopic characterization of TiNi nearly equiatomic shape memory alloys for implants [J]. J Vac Sci Technol, 1995, A13(5): 2624-2632.
[16] WEVER D J, VELDHUIZEN A G, DE VRIES J, BUSSCHER H J, UGES D R A, VAN HORN J R. Electrochemical and surface characterization of a nickel-titanium alloy [J]. Biomaterials, 1998, 19(7/9): 761-769.
[17] ARMITAGE D A, PARKER T L, GRANT D M. Biocompatibility and hemocompatibility of surface-modified NiTi alloys [J]. J Biomed Mater Res, 2003, A66(1): 129-137.
[18] LAMPIN M, WAROCQUIER-CLEROUT R, LEGRIS C, DEGRANGE M, SIGOT-LUIZARD M F. Correlation between substratum roughness and wettability, cell adhesion, and cell migration [J]. J Biomed Mater Res, 1997, 36(1): 99-108.
[19] TAKEBE J, ITOH S, OKADA J, ISHIKASHI K. Anodic oxidation and hydrothermal treatment of titanium results in a surface that causes increased attachment and altered cytoskeletal morphology of rat bone marrow stromal cells in vitro [J]. J Biomed Mater Res, 2000, 51(3): 398-407.
Foundation item: Project(50501007) supported by the National Natural Science Foundation of China; Project supported by Program for New Century Excellent Talents(NCET) in University of Ministry of Education of China; Project(7001999) supported by the SRG grant from the Research Committee of the CityU of HK; Project(4012001007) supported by the Teaching and Research Award Program for Outstanding Young Teachers of Southeast University, China
Corresponding author: CHU Cheng-lin; Tel: +86-25-52090683; E-mail: clchu@seu.edu.cn
(Edited by LI Xiang-qun)