双功能离子液体萃取剂[A336][P204]萃取Ce(IV)和F(I)的动力学
来源期刊:中国有色金属学报(英文版)2014年第6期
论文作者:杨华玲 陈 继 张冬丽 王 威 崔红敏 刘 郁
文章页码:1937 - 1945
Key words:Ce(IV)-F- system; Bif-ILE; kinetics model; extraction kinetics; constant interfacial area cell with laminar flow
摘 要:利用层流恒界面池研究在硫酸体系中双功能离子液体萃取剂[[A336][P204]]萃取Ce(IV)和F(I)的动力学过程,以便阐述萃取机理及萃取过程中的传质机制。在动力学数据分析中采用假一级反应动力学方法处理。通过讨论在Ce(IV)和Ce(IV)-F(I)的混合体系中搅拌速率、比界面积和温度对萃取的影响,推导出萃取过程是由水相和界面混合化学反应控制的。通过研究在Ce(IV)和Ce(IV)-F(I)的混合体系中各反应物对萃取速率常数的影响,从而确定了反应速率方程。通过机理和动力学模式的推导,验证了萃取反应速率方程的可靠性。
Abstract: The extraction kinetics of Ce(IV) and Ce(IV)-F- mixture systems from sulfuric solutions to n-heptane solution containing Bif-ILE [A336][P204] ([trialkylmethylammonium][di-2-ethylhewanxylphosphinate]) with a constant interfacial area cell with laminar flow were studied, just to elucidate the extraction mechanism and the mass transfer models. The data were analyzed in terms of pseudo-first-order constants. The effects of stirring speed, specific interfacial area and temperature on the extraction rate in both systems were discussed, suggesting that the extractions were mixed bulk phases-interfacial control process. Supported by the experimental data, the corresponding rate equations for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were obtained. The experimental results indicated the rate-controlling step. The kinetics model was deduced from the rate-controlling step and consistent with the rate equation.
Trans. Nonferrous Met. Soc. China 24(2014) 1937-1945
Hua-ling YANG1,2, Ji CHEN1, Dong-li ZHANG1, Wei WANG1, Hong-min CUI1, Yu LIU1
1. State Key Laboratory of Rare Earth Resources Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China;
2. School of Chemistry and Chemical Engineering, Nantong University, Nantong 226007, China
Received 18 July 2013; accepted 7 April 2014
Abstract: The extraction kinetics of Ce(IV) and Ce(IV)-F- mixture systems from sulfuric solutions to n-heptane solution containing Bif-ILE [A336][P204] ([trialkylmethylammonium][di-2-ethylhewanxylphosphinate]) with a constant interfacial area cell with laminar flow were studied, just to elucidate the extraction mechanism and the mass transfer models. The data were analyzed in terms of pseudo-first-order constants. The effects of stirring speed, specific interfacial area and temperature on the extraction rate in both systems were discussed, suggesting that the extractions were mixed bulk phases-interfacial control process. Supported by the experimental data, the corresponding rate equations for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were obtained. The experimental results indicated the rate-controlling step. The kinetics model was deduced from the rate-controlling step and consistent with the rate equation.
Key words: Ce(IV)-F- system; Bif-ILE; kinetics model; extraction kinetics; constant interfacial area cell with laminar flow
1 Introduction
Solvent extraction processes represent a significant technique in rare earths extraction and separation, as well as in industrial scale separation process [1,2]. As solvent or extractant in solvent extraction process [3-8], the quaternary ammonium ILs have recently attracted wide attention [5] due to their lower cost and toxicity, compared with other categories of ILs, i.e., imidazolium-type, pyrrolidinium-type, and phosphonium-type [9]. The pure bifunctional ionic liquid extractants (Bif-ILEs) by combining the cation of A336 (aliquat336/trialkylmethylammonium chloride) with phosphonic acid group or carboxylic acid group as anions, can be prepared by acid/base neutralization method. And the physical and chemical properties have been extensively researched [10]. Based on its potential advantages, such as low cost, good physico-chemical properties, low aqueous solubility, good resistance to hydrolysis and low extraction acidity, it has been systematically studied in the extraction of metal ions [11,12], especially for rare earths [13-15]. To access the extraction behavior of Bif-ILEs, our group also investigated the extraction and recovery of cerium(IV) (Ce(IV)) and fluoride (F-) from sulfuric solutions using Bif-ILE [A336][P204](tricaprymethylammonium di-2- ethylhexylphosphinate) [14]. During the extraction process, if using neutral Bif-ILE instead of the traditional acidic extractants, the extraction acidy will be much lower and this will help to reduce the extraction cost [16]. Moreover, the Bif-ILEs are easy to be synthesized and have good purity. The Bif-ILEs may highlight considerable opportunities in such fields [17].
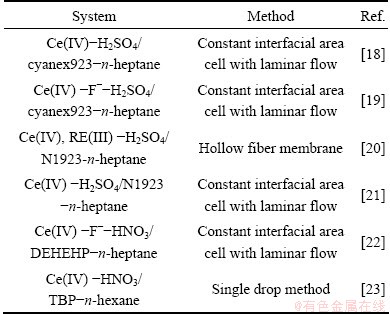
The extraction thermodynamics of rare earths with Bif-ILEs have been reported in our previous work [13,14]. However, there is no report on the extraction kinetics with Bif-ILEs. It is generally known that extraction kinetics research can provide useful information for optimizing processes, and is helpful in clarifying the mechanism of extraction. Hence, it is necessary to investigate the extraction kinetics of rare earths with Bif-ILEs and elucidate the extraction mechanism and mass transfer models. Table 1 shows the extraction kinetics study of Ce(IV) or Ce(IV) and F- from sulfuric acid or nitric acid solution. The extraction system of Ce(IV) or Ce(IV)-F- from bastnaesite has been concerned widely [24,25]. The theoretical research on the extraction thermodynamics and kinetics can provide reference for industry application. Therefore, as part of continuing work, the extraction kinetics of Ce(IV) and Ce(IV)-F- mixture system from sulfuric solutions using Bif-ILEs [A336][P204] was investigated following the thermodynamic investigation. Among the experimental equipments and techniques used in traditional extraction system, such as the single-drop technique, the constant interfacial area stirred cell and the rotating membrane cell, the constant interfacial area cell with laminar flow developed by ZHENG et al [26] has been used widely [18-22], owing to the stability and reproducibility of data.
Table 1 Methods of extraction of Ce(IV) or Ce(IV) and F- from sulfuric acid or nitric acid solution
Following the thermodynamic investigation, in this study, the extraction kinetics of Ce(IV) and Ce(IV)-F- mixture system from sulfuric acid solution using Bif-ILE [A336][P204] was investigated with a constant interfacial cell and laminar flow. Although this dynamic device still has its own drawbacks, this is the most effective method for the extraction systems to provide some reference data at present. The extraction mechanism and mass transfer models in extraction processes are proposed, providing more useful information for the application of the systems, especially for the advanced high-efficient clean processes. The reaction regimes deduced from the experimental results and the kinetics models were obtained from the rate- controlling step.
2 Experimental
2.1 Materials and reagents
Di-(2-ethyl hexyl) phosphate (95% purity, P204) was supplied by Tianjin Beicheng Chemical Plant (China) and used without further purification. Aliquat 336 (>99%) was purchased from Aldrich. The Bif-ILE [A336][P204] was synthesized in our lab according to published method [10]. The extractant was diluted with n-heptane. Stock solutions of Ce(IV) and F- were prepared by dissolving Ce(SO4)2·4H2O (>99.9%) into sulfuric acid and sodium fluoride into deionized water, respectively. Initial concentrations of F- and Ce(IV) were maintained at 0.02 mol/L and 0.01 mol/L for all the studies, respectively. Ce(IV) concentration before extraction was determined by titration with standard (NH4)2Fe(SO4)2 using o-phenanthroline as indicator. The concentration of Ce(IV) in aqueous phase after extraction was determined spectrophotometrically using a Shimadzu (Kyoto, Japan) UVmini-1240 UV-visible spectrophotometer, and the concentration in organic phase was obtained by mass balance. The concentration of F- was monitored by ion chromatography (Dionex ICS-1500, America) while the experiments on the effect of solution acidity were studied. The sulfuric acid concentration in aqueous phase was obtained by titrating with standard NaOH solution using phenolphthalein as indicator. The slope analysis was used to determine the extraction equations.
2.2 Apparatus and measurements
Kinetic experiments were carried out using a constant interfacial area cell modified with laminar flow by ZHENG et al [26] by immersing in a constant temperature water bath (DKB-501A, Senxin Co., Ltd., Shanghai). The schematic of the constant interfacial area cell with laminar flow is shown in Fig. 1.
Equal volume of the aqueous phase followed by organic phase was added carefully to the cell through injection hole for aqueous phase and organic phase using syringe, respectively, and stirring started at once. Both aqueous and organic phase volumes were 90.0 cm3. The organic phase was injected into the cell carefully in order to minimize the disturbance of the interface. The motors, digital overhead stirrers (IKA RW 20 digital), were employed for stirring. The rotation speed was adjusted with the rotary knob on the front plate and displayed on the LED display. Compared with the motors used in previous researches, the operating is more convenient (speed display: LED-Display) and the data obtained are more accurate and reliable in stability and reproducibility (the measurement fault: max. ±0.5%). As we know, it is important that the equipment has good stability, reproducibility and convenience for the kinetic research.
RW 20 digital), were employed for stirring. The rotation speed was adjusted with the rotary knob on the front plate and displayed on the LED display. Compared with the motors used in previous researches, the operating is more convenient (speed display: LED-Display) and the data obtained are more accurate and reliable in stability and reproducibility (the measurement fault: max. ±0.5%). As we know, it is important that the equipment has good stability, reproducibility and convenience for the kinetic research.
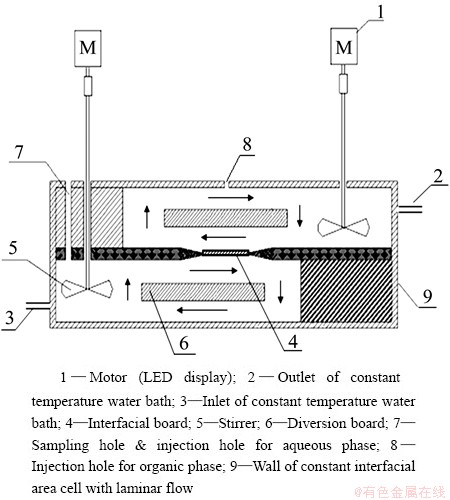
Fig. 1 Scheme of constant interfacial area cell with laminar flow
With starting the stirrer, the aqueous phase and the organic phase were in laminar flow in reverse at the action of diversion board, and the mass transfer process occurred on the interfacial board. After each 3 min interval, 0.3 mL of the aqueous phase was taken out through sampling hole for analysis. The interfacial area was 18.0 cm2 except specific interfacial area experiments. The kinetic experiments were carried out at 25 °C except the temperature experiments.
2.3 Data treatment
The experimental data were calculated following the theoretical formulas deduced by DANESI and VANDEGRIFT [27]. Assuming that the mass transfer process could be formally treated as a pseudo-first-order reversible reaction with respect to the metal cation:
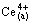

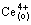 (1)
(1)
where (o) and (a) represent the organic phase and the aqueous phase, respectively. Thus the extraction rate is expressed as
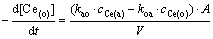 (2)
(2)
In Eq. (2), kao and koa are forward pseudo-first- order rate constant and backward pseudo-first-order rate constant, respectively; cCe(o) and cCe(a) are the concentrations of Ce(IV) in the organic phase and the aqueous phase at the time ‘t’. When the stirring speed is fixed, the forward pseudo-first-order rate constant kao and backward pseudo-first-order rate koa are functions of the concentration of [A336][P204] in the organic phase and the concentration of Ce(IV) in the aqueous phase.
By setting
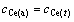 ,
, 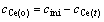
Considering that Eq. (2) is equal to zero at equilibrium, that is
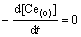
It follows:
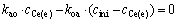
That is
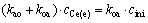 (3)
(3)
where (e) represents the equilibrium of metal ion. From Eq. (3), the relationship between kao and koa is as follows:

By integrating Eq. (2) and combing it with Eq. (3), we obtain
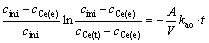 (4)
(4)
Or
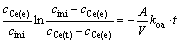 (5)
(5)
In the above expressions, A is the specific interfacial area and V is the volume of either the aqueous or organic phase. The function [(cini-cCe(e))/(cini)]ln[(cini-cCe(e))/ (cCe(t)-cCe(e))] versus time t was plotted for each experiment. The slopes of the plots were used to calculate kao and koa. All plots gave straight lines, indicating that the mass-transfer process could be treated as a pseudo–first–order reversible reaction with respect to the metal cation.
3 Results and discussion
3.1 Dependence of lgkao on stirring speed
The effects of the stirring speed of the two phases on forward extraction rates kao were studied for Ce(IV) and Ce(IV)-F- extraction systems, and the results are shown in Fig. 2. The extraction rate of Ce(IV) exhibited a kinetic plateau when the stirring speed was in the range from 350 to 450 r/min in Ce(IV) extraction system, and 250 to 350 r/min in Ce(IV)-F- extraction system, respectively. When the extraction rate increased continuously in Ce(IV)-F- extraction system, the wave was observed at the liquid-liquid mass transfer interface. In order to maintain the same hydrodynamic conditions, all other kinetic experiments were performed at 350 r/min in Ce(IV) extraction system and 250 r/min in Ce(IV)-F- extraction system, respectively. The data also indicated that the addition of F- decreased the extraction rate of Ce(IV). These results differed from those of Ce(IV) extraction from H2SO4 medium by Cyanex 923 [19], which showed that HF accelerated the Ce(IV) extraction rate.
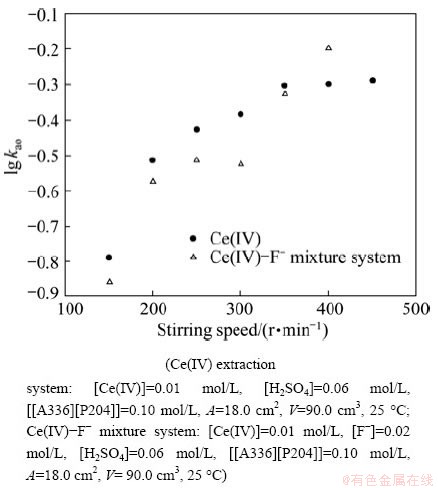
Fig. 2 Dependence of lg kao on stirring speed
In the extraction kinetic experiments, one of the criteria, applied to identifying the extraction regime, is the dependence of the extraction rate on the stirring speed in constant interfacial area cell with laminar flow. It can be a preliminary understanding of extraction kinetics model by examining the effect of stirring speed on extraction rate. In the mass transfer process, the extraction rate is always related to diffusion and kinetics reaction. The extraction rate is controlled by the kinetics reaction itself when the kinetics reaction rate is very slow. But it is controlled by the diffusion process when the reaction rate is very fast. Otherwise, it is controlled by both chemical reaction and diffusion process. In a constant interfacial cell, the relationship between the extraction rate and stirring speed was into a straight line in the early stage of the extraction reaction. The possible reason is that the stationary interfacial film is thicker at a lower stirring speed, and diffusion limits the rate of an extraction with a relatively fast chemical reaction. With the increase of the stirring speed, the interfacial film becomes thin and the diffusion resistance becomes small. Above a certain stirring speed, the increase of stirring speed will not increase extraction rate, and that is the ‘plateau region’. The presence of the ‘‘plateau region’’ in the extraction rate versus stirring speed curve indicated that the extraction rate might be kinetics controlled. However, a ‘‘plateau region’’ can be also generated by other phenomena. Even in extraction kinetics experiments the extraction rate was independent of the speed, the mass transfer process could be still diffusion controlled or at least not fully kinetics controlled. Therefore, it is necessary to identify the extraction regime with the help of other approach.
3.2 Dependence of lg kao on specific interfacial area
In order to know the rate-controlling step, the effect of specific interfacial area on the extraction rate was discussed. The effect of specific interfacial area on the extraction rate also can be used as one of the criteria for the rate-controlling step. If the reaction was controlled by diffusion control or mixed chemical reaction-diffusion control process, both stirring speed and specific interfacial area will affect the extraction rate. If the reaction is chemical reaction control, according to the reaction position, it would be bulk phase reaction control, interfacial reaction control or mixed bulk phase- interfacial reaction control. The distinction between the two types of reactions can be deduced from the effect of the specific interfacial area A/V (interfacial area/phase volume) on the initial extraction rate. The initial rate will be independent on interfacial area if the slow chemical reactions occur in the bulk phases. On the contrary, the reaction occurring at the interface will show a direct proportionality between the extraction rate and the interfacial area, and the straight line will be through the origin. In addition, other linear relationships will be regarded as mixed bulk phases-interfacial area control processes.
Figure 3 shows the liner relationship of lg kao versus A/V in Ce(IV) extraction system and Ce(IV)-F- mixture extraction system, and the liner relationships (y=0.0898+1.9601x in Ce(IV) extraction system and y=0.0658+2.7565x in Ce(IV)-F- mixture extraction system, respectively) were obtained. The results indicated that the reactions may be mixed bulk phases-interfacial area control processes. The Ce(IV) system gave the higher extraction rate, while the Ce(IV)-F- mixture system gave the lower extraction rate.
3.3 Effect of temperature on extraction rate
The effect of temperature on the extraction rate of chemical reaction control was significant, but a little effect on diffusion control process. Generally, when the activation energy (Ea) of an extraction is more than 42 kJ/mol, the extraction process is controlled by chemical reaction. When Ea is lower than 20 kJ/mol, species diffusion is rate-limiting step. The extraction rate is determined by both chemical reaction and diffusion when Ea is in the range from 20 to 42 kJ/mol.
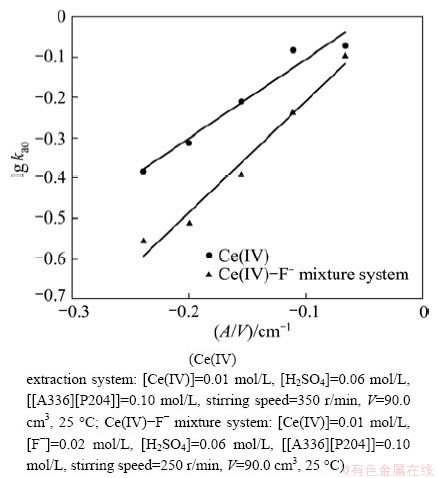
Fig. 3 Dependence of lg kao on specific interfacial area
The effect of temperature on the extraction rate was studied for the temperature in the range from 20 to 45 °C. The results are shown in Fig. 4. It is found that the extraction rate decreased with the increase of temperature in both systems. The extraction activation energy was obtained from the slope of lg kao versus 1000/T according to the Arrhenius equation:
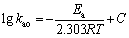 (6)
(6)
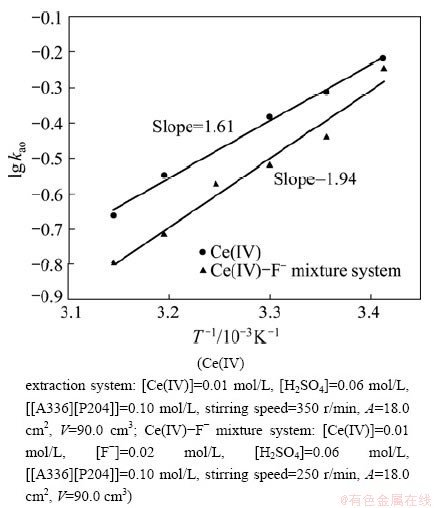
Fig. 4 Dependence of rate constants on temperature
where C is the rate constant, and Ea is the activation energy.
The values of Ea for the forward reaction Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were -30.85 kJ/mol and -37.05 kJ/mol, respectively. The results indicated that both of the extraction of Ce(IV) and Ce(IV)-F- were controlled by chemical reaction-diffusion processes, and the addition of F- increased the activation energy. This probably results in the extraction rate of Ce(IV) extraction system higher than that of Ce(IV)-F- mixture extraction system. However, DANESI and VANDEGRIFT [27] reported that Ea was not sufficient for the judgement of the kinetics process in the multi-component chemical reaction system. Therefore, several comprehensive analysis ways are needed for the determination of the control procedures. In fact, if the rate is controlled by a chemical reaction, Ea is usually higher than the diffusion control. However, Ea values of many chemical solvent extraction processes are only a few kJ/mol, that is, the magnitude order is the same with Ea of the diffusion process. So this method is not very strict.
3.4 Effect of concentration of Bif-ILE [A336][P204] in organic phase on extraction rate
The effect of the Bif-ILE [A336][P204] concentration on the extraction rate was investigated in Ce(IV) extraction system and Ce(IV)-F- mixture extraction system. As shown in Fig. 5, the slopes of the plots for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were 0.47 and 0.37, respectively. The results indicated that the reaction orders of Bif-ILE [A336][P204] in these systems were 0.47 and 0.37, respectively.

Fig. 5 Effect of [A336][P204] concentrations on extraction rate kao
3.5 Effect of salting-out agent concentration on extraction rate
The effect of salting-out agent concentration on the extraction rate was investigated. Keeping other conditions unchanged, different amount of salting-out agent Na2SO4 or NaHSO4 was added to the aqueous solutions. The concentration of salting-out agent was much higher than other reagents, and thus the ionization and hydrolysis of corresponding weak acid salt could be ignored in data measurement. So the added concentrations of  and
and  were considered as concentration of
were considered as concentration of  and
and  in the solution, respectively, at a constant acidity.
in the solution, respectively, at a constant acidity.
3.5.1 Effect of  concentration
concentration
Figure 6 shows the influence of the  concentration on the extraction rate in Ce(IV) extraction system and Ce(IV)-F- mixture extraction system. The slopes of the plots for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were -1.43 and -0.95, indicating that the reaction orders of
concentration on the extraction rate in Ce(IV) extraction system and Ce(IV)-F- mixture extraction system. The slopes of the plots for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were -1.43 and -0.95, indicating that the reaction orders of  in these systems were -1.43 and -0.95, respectively. The negative order also indicated that the extraction rate decreased with the increase of
in these systems were -1.43 and -0.95, respectively. The negative order also indicated that the extraction rate decreased with the increase of  concentration in the both systems.
concentration in the both systems.
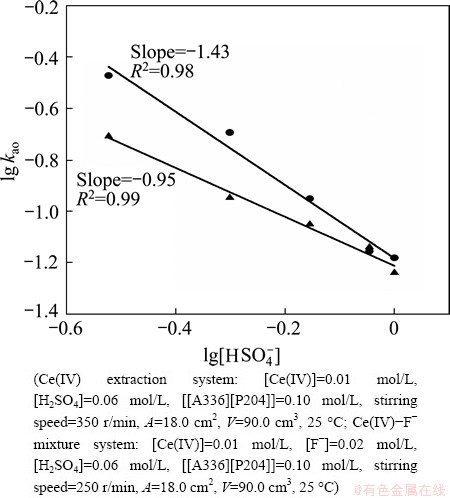
Fig. 6 Effect of [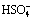 ] concentrations on extraction rate, kao
] concentrations on extraction rate, kao
3.5.2 Effect of 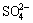 concentration on extraction rate
concentration on extraction rate
As shown in Fig. 7, the effects of 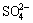 concentration on the extraction rate in Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were also investigated. The slopes of the plots for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were -0.77 and -0.85, respectively, which indicated that the reaction orders of
concentration on the extraction rate in Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were also investigated. The slopes of the plots for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were -0.77 and -0.85, respectively, which indicated that the reaction orders of 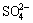 in these systems were -0.77 and -0.85, respectively. The negative order also indicated that the increasing
in these systems were -0.77 and -0.85, respectively. The negative order also indicated that the increasing 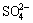 concentration directly decreased the extraction rate in both systems.
concentration directly decreased the extraction rate in both systems.
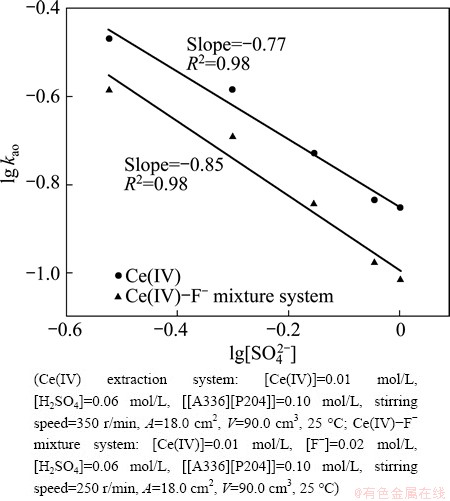
Fig. 7 Effect of [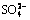 ] concentrations on extraction rate, kao
] concentrations on extraction rate, kao
The negative order of both 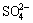 and
and  indicated that the extraction rate decreased with the increasing of
indicated that the extraction rate decreased with the increasing of 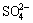 and
and  concentrations in both systems, which was consistent with the results that increasing
concentrations in both systems, which was consistent with the results that increasing 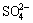 and
and  concentration decreased the distribution ratio of Ce(IV) and CeF3+ [14]. The stabilities of Ce(IV) and Ce(IV) complex with F- in solution were reported [28], and the complex form of Ce(IV) with F- was
concentration decreased the distribution ratio of Ce(IV) and CeF3+ [14]. The stabilities of Ce(IV) and Ce(IV) complex with F- in solution were reported [28], and the complex form of Ce(IV) with F- was 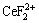 , CeF3+ or CeF4 in solution at different Ce(IV) concentration and acidity. In our previous work, under the selected condition, Ce(IV) and F- were extracted in the form of CeF3+ [29]. Therefore, as continuous work, the selected experiment condition was consistent with our previous work. The effect of F- concentration and the complex form of Ce(IV) with F- considered as CeF3+ were not discussed further in this work.
, CeF3+ or CeF4 in solution at different Ce(IV) concentration and acidity. In our previous work, under the selected condition, Ce(IV) and F- were extracted in the form of CeF3+ [29]. Therefore, as continuous work, the selected experiment condition was consistent with our previous work. The effect of F- concentration and the complex form of Ce(IV) with F- considered as CeF3+ were not discussed further in this work.
The plots in Figs. 2, 3, 4, 5, 6 and 7 also show that the extraction rate of Ce(IV)-F- mixture extraction system is far less than that of Ce(IV) extraction system, and the results are consistent with the previous report [22]. According to the experiment results, the rate equations for Ce(IV) and Ce(IV)-F- mixture extraction system with Bif-ILE [A336][P204] can therefore be expressed as
-d[Ce(IV)](a)/dt=k[Ce(IV)](a)·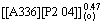 ·
· ·
·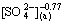 (7)
(7)
-d[CeF3+](a)/dt=k[CeF3+](a)·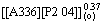 ·
·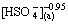 ·
·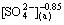 (8)
(8)
4 Mechanism and kinetic model
On the basis of the above discussion, the possible mechanism and kinetic model in the extraction process were controlled by mixed chemical reaction-diffusion processes in terms of a kinetic extraction regime, and we proposed the extraction mechanism of Ce(IV) with Bif-ILE [A336][P204] as follows:
B(o) B(i) (9)
B(i) (9)
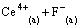
 (10)
(10)
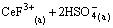

 (11)
(11)

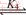
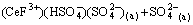 (12)
(12)
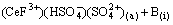

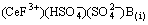 (13)
(13)
So,

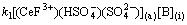 (14)
(14)

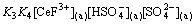 (15)
(15)
where B presents Bif-ILE [A336][P204]; K1, K2, K3 and K4 present equilibrium constants, respectively; k1 and k-1 present forward and backward reaction rate constants, respectively; “i”, “o” and “a” represent interfacial, organic and aqueous phase, respectively.
In the aqueous phase, the equilibrium takes place:
Ce4++F- CeF3+ (16)
CeF3+ (16)
It can be known that Ce(IV) mainly exists in the form of CeF3+ in the aqueous phase under the experimental condition, that is
[Ce4+]t≈[CeF3+] (17)
From Eq. (9), the following equation could be obtained
[B](i)=K1[B](o) (18)
Assuming Eq. (13) is the rate-controlling step, and the following equation can be written as
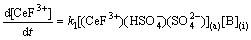 (19)
(19)
Introducing Eqs. (10), (11) and (18) into (19), then the following expression was obtained,
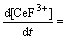
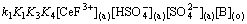 (20)
(20)
The extraction rate equation can be written as follows:
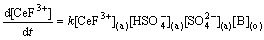 (21)
(21)
where k=k1K1K3K4.
Equation (21) deduced from the rate controlling- step exhibits good agreement with the rate equation obtained from the experimental results. The non-integer value of the reaction order could be explained by the non-ideal behavior of the solutes participating in the reaction or another competing reaction.
5 Conclusions
1) The extraction kinetics of Ce(IV) and Ce(IV)-F- mixture system from H2SO4 solution to n-heptane solutions containing Bif-ILE [A336][P204] was investigated using a constant interfacial cell with laminar flow. According to the experimental data, the corresponding rate equations for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were deduced as follows:
-d[Ce(IV)](a)/dt=k[Ce(IV)](a)·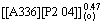 ·
· ·
·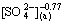
-d[CeF3+](a)/dt=k[CeF3+](a)·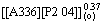 ·
·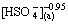 ·
·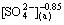
2) By investigating the effects of stirring speed, specific interfacial area and temperature on the extraction rate, it was concluded that the extractions for Ce(IV) extraction system and Ce(IV)-F- mixture extraction system were mixed bulk phases-interfacial area control processes.
3) The extraction rate of Ce(IV)-F- mixture extraction system was less than that of Ce(IV) extraction system.
References
[1] LI Xing-bin, WEI Chang, WU Jun, LI Cun-xiong, LI Min-ting, DENG Zhi-gan, XU Hong-sheng. Thermodynamics and mechanism of vanadium (IV) extraction from sulphate medium with D2EHPA, EHEHPA and CYANEX 272 in kerosene [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 461-466.
[2] FU Nian-xin, SUI Zhi-tong, MIKIYA T. Equilibrium analysis of solvent extraction of yttrium (III) and europium (III) from hydrochloric acid with P507 [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2093-2098.
[3] DIETZ M L, STEPINSKI D C. A ternary mechanism for the facilitated transfer of metal ions into room-temperature ionic liquids (RTILs): Implications for the “greenness” of RTILs as extraction solvents [J]. Green Chemistry, 2005, 7: 747-750.
[4] TIAN Guo-cai, LI Jian, HUA Yi-xin. Application of ionic liquids in hydrometallurgy of nonferrous metals [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 513-520.
[5] EGOROV V M, DJIGAILO D I, MOMOTENKO D S, CHERNYSHOV D V, TOROCHESHNIKOVA I I, SMIRNOVA S V, PLETNEV I V. Task-specific ionic liquid trioctylmethylammonium salicylate as extraction solvent for transition metal ions [J]. Talanta, 2010, 80: 1177-1182.
[6] YANG Xiu-yun, ZHANG Jian-ping, GUO Lin, ZHAO He, ZHANG Yang, CHEN Ji. Solvent impregnated resin prepared using ionic liquid Cyphos IL 104 for Cr(VI) removal [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 3126-3130.
[7] PEREIRO A B, RODRIGUEZ A. An ionic liquid proposed as solvent in aromatic hydrocarbon separation by liquid extraction [J]. AIChE Journal, 2010, 56: 381-386.
[8] BELL T J, IKEDA Y. The application of novel hydrophobic ionic liquids to the extraction of uranium(VI) from nitric acid medium and a determination of the uranyl complexes formed [J]. Dalton Transactions, 2011, 40: 10125-10130.
[9] LIU Ying-hui, CHEN Ji, LI De-qian. Application and perspective of ionic liquids on rare earths green separation [J]. Separation Science and Technology, 2012, 47: 223-232.
[10] SUN Xiao-qi, JI Yang, LIU Yu, CHEN Ji, LI De-qian. An engineering-purpose preparation strategy for ammonium-type ionic liquid with high purity [J]. AIChE Journal, 2010, 56: 989-996.
[11] SUN Xiao-qi, JI Yang, ZHANG Li-na, CHEN Ji, LI De-qian. Separation of cobalt and nickel using inner synergistic extraction from bifunctional ionic liquid extractant (Bif-ILE) [J]. Journal of Hazardous Materials, 2010, 182: 447-452.
[12] LIU Ying-hui, GUO Lin, CHEN Ji. Removal of Cr(III, VI) by quaternary ammonium and quaternary phosphonium ionic liquids functionalized silica materials [J]. Chemical Engineering Journal, 2010, 158: 108-114.
[13] WANG Wei, YANG Hua-ling, CUI Hong-min, ZHANG Dong-li, LIU Yu, CHEN Ji. Application of bifunctional ionic liquid extractants [A336][CA-12] and [A336][CA-100] to the lanthanum extraction and separation from rare earths in the chloride medium [J]. Industrial & Engineering Chemistry Research, 2011, 50: 7534-7541.
[14] ZHANG Dong-li, WANG Wei, DENG Yue-feng, ZHANG Jian-ping, ZHAO He, CHEN Ji. Extraction and recovery of cerium(IV) and fluorine(I) from sulfuric solutions using bifunctional ionic liquid extractants [J]. Chemical Engineering Journal, 2012, 179: 19-25.
[15] YANG Hua-ling, WANG Wei, CUI Hong-min, ZHANG Dong-li, LIU Yu, CHEN Ji. Recovery of rare earth elements from simulated fluorescent powder using bifunctional ionic liquid extractants (Bif-ILEs) [J]. Journal of Chemical Technology and Biotechnology, 2012, 87: 198-205.
[16] GUPTA C K, KRISHNAMURTHY N. Extractive metallurgy of rare earths [M]. Washington, D.C.: CRC Press, 2004: 183-504.
[17] WELLENS S, THIJS B, BINNEMANS K. An environmentally friendlier approach to hydrometallurgy: Highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids [J]. Green Chemistry, 2012, 14: 1657-1665.
[18] YU Gui-hong, YUE Shan-tang, LI De-qian, YOU Feng-you. Kinetic study of Ce4+ extraction with Cyanex 923 [J]. Journal of Rare Earths, 2001, 19(4): 250-254.
[19] LIAO Wu-ping, YU Gui-hong, YUE Shan-tang, LI De-qian. Kinetics of cerium(IV) extraction from H2SO4-HF medium with Cyanex 923 [J]. Talanta, 2002, 56: 613-618.
[20] WANG Yu-jie, ZHANG Shu-min, LI De-qian. Separation of Th4+ and RE3+ with hollow fiber membrane extraction [J]. Journal of Rare Earths, 1998, 16(3): 193-198.
[21] LIU Jian-jun, WANG Yan-liang, LI De-qian. Extraction kinetics of cerium(IV) from sulfuric acid medium by the primary amine N1923 using a constant interfacial area cell with laminar flow [J]. Journal of Chemical Technology and Biotechnology, 2007, 82: 949-955.
[22] ZHAO Jun-mei, LI Wei, LI De-qian, XIONG Ying. Kinetics of cerium(IV) extraction with DEHEHP from HNO3-HF medium using a constant interfacial cell with laminar flow [J]. Solvent Extraction and Ion Exchange, 2006, 24: 165-176.
[23] QIN Qi-zong, ZHOU Zu-ming, YANG Yong-le, TANG You-hong, LI Gui-hua, XI Yong-de. Kinetic studies on the solvent extraction (I)— Extraction rate and mechanism of cerium(IV) nitrate in the TBP-HNO3 system [J]. Journal of Fudan University, 1981, 20(1): 148-151. (in Chinese)
[24] FENG Xing-liang, LONG Zhi-qi, CUI Da-li, WANG Liang-shi, HUANG Xiao-wei, ZHANG Guo-cheng. Kinetics of rare earth leaching from roasted ore of bastnaesite with sulfuric acid [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 849-854.
[25] WANG Liang-shi, YU Ying, HUANG Xiao-wei, LONG Zhi-qi, CUI Da-li. Toward greener comprehensive utilization of bastnaesite: Simultaneous recovery of cerium, fluorine, and thorium from bastnaesite leach liquor using HEH(EHP) [J]. Chemical Engineering Journal, 2013, 215-216: 162-167.
[26] ZHENG Zhong, LU Jun, LI De-qian. The kinetics study in liquid-liquid systems with constant interfacial area cell with laminar flow [J]. Chemical Engineering Science, 1998, 53: 2327-2333.
[27] DANESI P R, VANDEGRIFT G F. Kinetics and mechanism of the interfacial mass transfer of europium(3+) and americium(3+) in the system bis(2-ethylhexyl)-phosphate-n-dodecane-sodium chloride- hydrochloric acid-water [J]. Journal of Chemical Physics, 1981, 39: 1425-1432.
[28] QIAO Jun, ZHANG Cun-rui, LIU Zhao-gang, HAO Xian-ku. Complexation behavior of fluorine(I) with cerium(IV) in solution [J]. Chinese Rare Earths, 1997, 18(3): 64-67. (in Chinese)
[29] ZHU Guo-cai, TIAN Jun, CHI Ru-an, XU Sheng-ming, ZHANG Zhi-geng. Recovering RE with NH4Cl roasting from bastnasite crude ore [J]. The Chinese Journal of Nonferrous Metals, 2000, 10(5): 701-704. (in Chinese).
杨华玲1,2,陈 继1,张冬丽1,王 威1,崔红敏1,刘 郁1
1. 中国科学院 长春应用化学研究所,稀土资源利用国家重点实验室,长春 130022;
2. 南通大学 化学化工学院,南通 226007
摘 要:利用层流恒界面池研究在硫酸体系中双功能离子液体萃取剂[[A336][P204]]萃取Ce(IV)和F(I)的动力学过程,以便阐述萃取机理及萃取过程中的传质机制。在动力学数据分析中采用假一级反应动力学方法处理。通过讨论在Ce(IV)和Ce(IV)-F(I)的混合体系中搅拌速率、比界面积和温度对萃取的影响,推导出萃取过程是由水相和界面混合化学反应控制的。通过研究在Ce(IV)和Ce(IV)-F(I)的混合体系中各反应物对萃取速率常数的影响,从而确定了反应速率方程。通过机理和动力学模式的推导,验证了萃取反应速率方程的可靠性。
关键词:Ce(IV)-F-;双功能离子液体萃取剂;动力学模型;萃取动力学;层流恒界面池
(Edited by Hua YANG)
Foundation item: Project (2012CBA01202) supported by the National Basic Research Program of China; Project (51174184) supported by the National Natural Science Foundation of China; Project (KGZD-EW-201-1) supported by the Key Research Program of the Chinese Academy of Sciences; Project (BK2013030) supported by Science and Technology Plan of Nantong City, China; Project (RERU2014016) supported by Open Subject of Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, China
Corresponding author: Ji CHEN; Tel: +86-431-85262646; E-mail: jchen@ciac.ac.cn
DOI: 10.1016/S1003-6326(14)63274-X

