Action rules of H2 and CO in gas-based direct reduction of iron ore pellets
来源期刊:中南大学学报(英文版)2012年第8期
论文作者:易凌云 黄柱成 彭虎 姜涛
文章页码:2291 - 2296
Key words:iron ore pellets; coal gas; gas-based direct reduction; reduction kinetics; gas utilization
Abstract: The gas-based direct reduction of iron ore pellets was carried out by simulating the typical gas composition in coal gasification process, Midrex and Hyl-III processes. The influences of gas composition and temperature on reduction were studied. Results show that the increasing of H2 proportion is helpful to improve the reduction rate. However, when φ(H2):φ(CO)>1.6:1, changes of H2 content have little influence on it. Appropriate reduction temperature is about 950 ℃, and higher temperature (1 000 ℃) may unfavorably slow the reduction rate. From the kinetics analysis at 950 ℃, the most part of reduction course is likely controlled by interfacial chemical reaction mechanism and in the final stage controlled by a combined effect of gaseous diffusion and interfacial chemical reaction mechanisms. From the utilizations study of different reducing gases at 950 ℃, the key step in reduction course is the 3rd stage (FeO→Fe), and the utilization of reducing gas increases with the rise of H2 proportion.
J. Cent. South Univ. (2012) 19: 2291-2296
DOI: 10.1007/s11771-012-1274-0![]()
YI Ling-yun(易凌云)1, HUANG Zhu-cheng(黄柱成)1, PENG Hu(彭虎)2, JIANG Tao(姜涛)1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Changsha Syno-Therm Co., Ltd., Changsha 410126, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: The gas-based direct reduction of iron ore pellets was carried out by simulating the typical gas composition in coal gasification process, Midrex and Hyl-III processes. The influences of gas composition and temperature on reduction were studied. Results show that the increasing of H2 proportion is helpful to improve the reduction rate. However, when φ(H2):φ(CO)>1.6:1, changes of H2 content have little influence on it. Appropriate reduction temperature is about 950 ℃, and higher temperature (1 000 ℃) may unfavorably slow the reduction rate. From the kinetics analysis at 950 ℃, the most part of reduction course is likely controlled by interfacial chemical reaction mechanism and in the final stage controlled by a combined effect of gaseous diffusion and interfacial chemical reaction mechanisms. From the utilizations study of different reducing gases at 950 ℃, the key step in reduction course is the 3rd stage (FeO→Fe), and the utilization of reducing gas increases with the rise of H2 proportion.
Key words: iron ore pellets; coal gas; gas-based direct reduction; reduction kinetics; gas utilization
1 Introduction
Compared with “blast furnace-converter-continuous casting” process, the “electric arc furnace-continuous casting” process with advantages of lower investment and energy consumption, higher productivity and better environmental protection is concerned by more and more steel enterprises. Due to the pure and stable composition, direct reduction iron (DRI) is considered to be the perfect material for electric arc furnace (EAF) steelmaking [1]. As EAF steel output grows and the high quality scrap supply is seriously insufficient, the demand for DRI will grow continuously in the world. The gas-based reduction process with advantages of mature technology and high productivity is the main one in direct reduction process. But using natural gas as reductant makes its development restricted by gas resource and region [2]. So, developing the coal gasification-gas based reduction process is significant for the promotion of steel industry in China.
The H2 and CO contents in reducing gas of Midrex and Hyl differ widely. However, the difference also exists in several mainstream of coal gasification process. KRZYSZTOF et al [3] investigated the effect of gas composition on the kinetics of iron oxide reduction and concluded that the Fe2O3→FeO reduction was proved to be initially a topochemical surface-controlled process, and once a thin layer of lower iron oxides was formed on the surface, mechanism clearly shifts to diffusion control. Studies of gas reduction mechanism of microscale iron oxide powders revealed that, in the initial stage, reaction is controlled by chemical reaction then by combination of chemical reaction and gas mass diffusion [4-5]. WANG et al [6] found that reduction rate increased with the increase of temperature and hydrogen content, and the rate controlling step was the diffusion of hydrogen gas in the reduced layer. Kinetic of pellets reduction in shaft furnace by two different reducing gases was previously studied [7-8]. It is proved that H2 can improve the dynamic condition of reducing gas. However, researches about the action rules of H2 and CO in direct reduction and the competition between the gas composition and the reduction process are rare. In this work, the direct reduction of iron ore pellets was carried out by simulating the typical gas composition in coal gasification process, Midrex and Hyl-III processes, and the influences of gas composition and temperature on reduction were studied.
2 Experimental
The chemical composition of iron ore pellets investigated in this work is listed in Table 1. The majority of the charged pellets were in the size range of 12.5-16 mm, and the crushing strength was 2 973 N per pellet. The temperature of reducing gas was 850-900 ℃ in Midrex process, and 930-950 ℃ in Hyl-III process. Gas composition in the two processes is listed in Table 2. Typical gas composition in coal gasification process is listed in Table 3 [9-10].
Table 1 Chemical composition of iron ore pellets (mass fraction, %)
![]()
Table 2 Gas composition in Midrex and Hyl-III processes (volume fraction, %)

Table 3 Gas composition of coal gasification process (volume fraction, %)

In Texaco and Shell processes, as in Midrex and Hyl processes, the total content of H2 and CO is about 90%, but the ratio of each differs widely. In Texaco process, the ratio of φ(H2):φ(CO) reaches about 1:1. In Shell, Midrex and Hyl processes, the ratios reach about 0.4, 1.6 and 2.6, respectively. So, compositions of the four reducing gases investigated in this work are given in Table 4, the ratios of φ(H2):φ(CO) are 0.4, 1.0, 1.6 and 2.6, respectively.
Table 4 Composition of reducing gas (volume fraction, %)
The schematic diagram of experimental apparatus is shown in Fig. 1 [11]. The prepared pellets were then reduced in a thermo gravimetric apparatus at 850– 1 050 ℃ using various reducing gases. The reduction setup and gas ?ow system used in this work were previously mentioned. The course of reduction was followed by measuring the mass loss as a function of time under controlled conditions of temperature and gas composition. For each reduction experiment, the furnace was heated to the required reduction temperature, and then the pellets were weighed and placed in the silica tube. The sample was then gradually introduced into the furnace so as to avoid thermal shock cracking and was positioned in the middle of constant hot zone in the furnace. First, nitrogen at a ?owrate of 1 L/min was introduced. Then, after sample soaking for 10 min at the reduction temperature the reducing gas at a ?owrate of 3 L/min was introduced. The mass loss resulted by oxygen removal from the pellets was recorded over time. At the end of the experiment, the silica tube with the reduced pellets was removed and cooled in atmosphere of nitrogen.
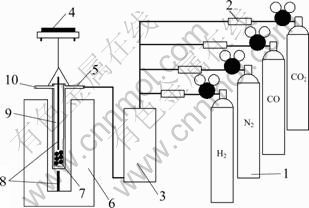
Fig. 1 Schematic diagram of apparatus: 1—Cylinder; 2—Gas flowmeter; 3—Mixer; 4—Electronic balance; 5—Gas inlet; 6—Sharft furnace; 7—Pellets; 8—Thermocouple; 9—Silica tube; 10—Gas outlet
3 Results and discussion
3.1 Effect of reducing gas composition
Iron ore pellets were reduced at 850-1 050 ℃ with the reducing gas proportion of H2:CO varying from 0.4 to 2.6. Changes of the reduction rate of the pellets with time during the reduction are shown in Fig. 2.
In general, the increase in hydrogen content caused a visible increase in reduction rate. As shown in Fig. 2, when the ratio of φ(H2):φ(CO) increases from 0.4 to 1.6, reduction rate increases obviously. In ratio range from 1.6 to 2.6, the reduction curves are almost coincident, which indicates that change of H2 content has little effect on the reduction rate. Due to the superior diffusion coefficient and adsorption capacity, H2 can improve the reduction rate [12]. However, in actual reduction process, reduction rate is also influenced by many other factors, such as temperature, ore size, porosity and reaction pressure. Reduction rate does not increase infinitely along with the raise of H2 content, so it is important for the optimization of reduction process to choose an appropriate composition of reducing gas.
3.2 Effect of temperature
The isothermal reduction curves at 850-1 050 ℃ are shown in Fig. 3. It was found that for each reduction curve, the rate of reduction increased rapidly at the early stages and decreased as reduction proceeds. In general, reduction rate increases with temperature. The reduction rate at 850 ℃ is slower compared to other reduction temperatures. However, with temperature up to 1 000 ℃, reduction rate significantly slows down to the same level as 850-900 ℃.
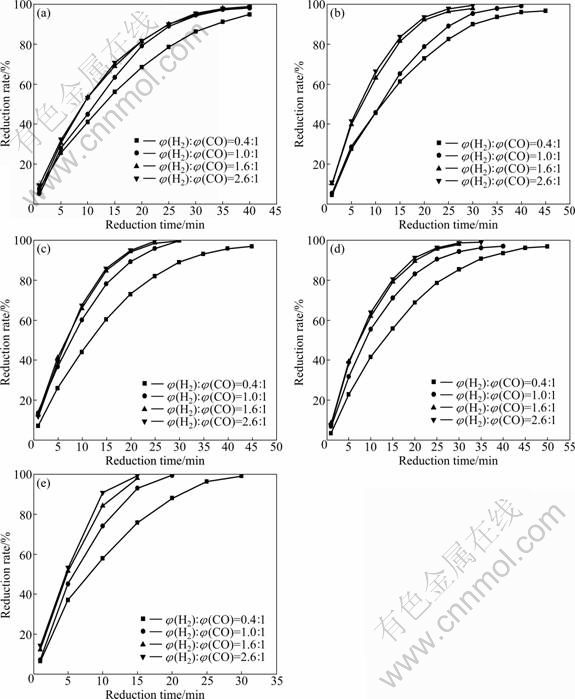
Fig. 2 Reduction rate of pellets by reducing gases with various ratios of H2:CO at 850 ℃ (a), 900 ℃ (b), 950 ℃ (c), 1 000 ℃ (d) and 1 050 ℃ (e)
Normally, rise of temperature can improve reduction reaction rate, but the porosity of iron ore in reduction process constantly changes. At lower temperature the change is very small, but at higher temperature, with the mineralogical transformation, sintering and softening occur in the pellets. Therefore, reduction rate and temperature curves appear special changes. LI [8] also found that pellets began to soften around 1 000 ℃, which hindered the reducing gas diffusion. On the other hand, the equilibrium constant of reaction increases with temperature:
H2+CO2=CO+H2O(g)+46.23 kJ/mol,
ln K=4.862-5561/T (1)
So, the concentration of H2 in the reaction system decreases, while that of CO and H2O increases, which results in reduction rate slowing. USUI et al [13] also proved the influence of reaction (Eq. (1)) that reduction rate at 900 ℃ was higher than that at 1 000 ℃.
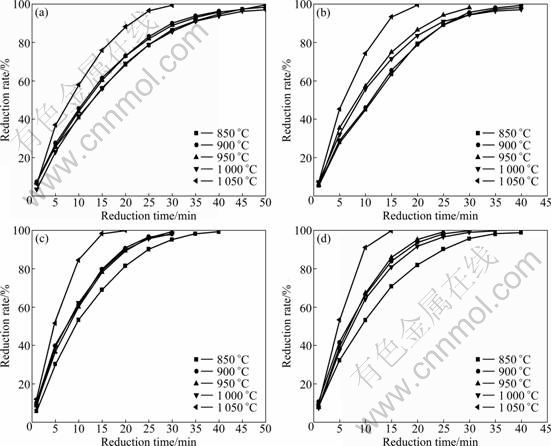
Fig. 3 Reduction rate of pellets at 850-1 050 ℃ by reducing gases with various ratios of φ(H2):φ(CO): (a) φ(H2):φ(CO)=0.4:1; (b) φ(H2):φ(CO)=1:1; (c) φ(H2):φ(CO)=1.6: 1; (d) φ(H2):φ(CO)=2.6: 1
3.3 Reaction kinetics
Based on the observed results, it can be concluded that the reduction of the pellet proceeded topochemically. This kind of reaction included the process of diffusion of gaseous species and that of intrinsic chemical reaction. The total reduction time is expressed by Eq. (2), which is valid under the conditions of solid sphere being reacted with gas phase, the effect of gas phase mass transfer being negligible and the reaction rate being controlled by intrinsic interfacial chemical reaction and the diffusion of reactant and product gas species through solid product layer [6, 14-15]:
![]()
![]() (2)
(2)
where t is reduction time (min); c0 and cq are reduction gas concentrations at granule surface and in equilibrium, respectively (mol/cm3); k is reaction rate constant (cm/min); De is effective diffusion coefficient (cm2/min); r0 is characteristic initial radius of pellets (cm); ρ0 is initial oxygen concentration in the pellet (mol/cm3); R is reduction degree.
Based on the results shown in Figs. 2 and 3, the reduction at 950 ℃ was optimum. So, applying Eq. (2), three cases for rate controlling step at 950 ℃ with various ratios of H2:CO were examined, namely, chemical reaction control, diffusion control and mixed control. The relationship between 1-(1-R)1/3 and time is shown in Fig. 4.
It was found that for the most part of reduction process (>90%), interfacial chemical reaction was the rate controlling step under all experimental conditions for gas content. In the last stage (<10%), diffusion of reducing gas in the reduced layer became the controlling step. In addition, with the ratio of φ(H2):φ(CO) changing, the endpoint of reaction rate control step was different. When the ratio of φ(H2):φ(CO) was 0.4, the former 91.36% (volume fraction) of reduction process was controlled by reaction rate step. However, when the ratio reached 1.0, 1.6 and 2.6, the part controlled by reaction rate step reached 96.51%, 98.53% and 99.59% (volume fraction), respectively. Obviously, H2 caused the decrease of the diffusion resistance due to the smaller molecule diameter. So, with the increase of H2 content, the diffusion control step in the last stage was significantly shortened.
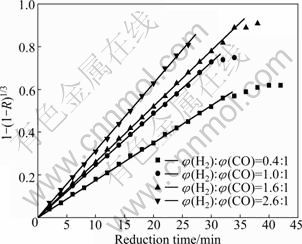
Fig. 4 Relationship between 1-(1-R)1/3 and time at 950 ℃
The slopes of lines in the figure were obtained from linear regression analysis, and the reaction rate constant was calculated under each experimental condition, as given in Table 5. As can be seen, interface reaction rate constant increases with the proportion rise of H2 in reducing gas. So, raising the proportion of H2 can effectively improve the reduction process.
Table 5 Reaction rate constant at 950 ℃ with various ratios of φ(H2):φ(CO)

3.4 Utilization analysis of reducing gas
The utilization of reducing gas with various ratios of H2:CO at 950 ℃ was studied, which was defined as [16]
![]() (3)
(3)
where x in Eq. (3) is the mole fractions of different components in exhaust. Similarly, the utilizations of CO and H2 were defined as
![]()
In shaft furnace, with the rise of temperature, reduction of Fe2O3 in iron ore experiences the following stages:
The first stage, Fe2O3→Fe3O4:
3Fe2O3+CO=2Fe3O4+CO2, ![]() (4)
(4)
3Fe2O3+H2=2Fe3O4+H2O, ![]() (5)
(5)
The second stage, Fe3O4→FeO:
Fe3O4+CO=3FeO+CO2, ![]() (6)
(6)
Fe3O4+H2=3FeO+H2O, ![]() (7)
(7)
The third stage, FeO→Fe:
FeO+CO=Fe+CO2, ![]() (8)
(8)
FeO+H2=Fe+H2O, ![]() (9)
(9)
Different stages of reduction require different gas conditions, and always the third stage requires the most reductant. Due to the thermal effects in the reduction of iron oxide, considering the thermodynamics, the requirement of reducing gas condition is relevant with temperature. So, the main thermodynamics factors influence the reduction of iron oxides are the composition of reducing gas, reduction stage and temperature. In the shaft furnace, exhaust of the third stage is reductant in the second stage, and the same with the second and first stages. So, gas utilization in this work refers to the utilization after completion of all three stages mentioned above.
In the isothermal reduction of Fe2O3, there is one stage that may reach equilibrium with reducing gas, which is the key stage of reduction process. So, the minimum requirement and utilization of reducing gas depend on the actual requirement and thermodynamics efficiency of the key stage. In reduction process, only the second or third stage may become the key stage. And the main effect factors on key stage are temperature and gas composition.
Utilization of the second stage:
![]()
Utilization of the third stage:
![]()
When generate 1 mol Fe, it will consume 1 mol reductant in the third stage. For the second stage, when
Fe3O4 is reducted to FeO, ![]() reductant will be
reductant will be
needed. So, in order to ensure the reductant need of the third stage, the minimum reductant supply can be expressed as M3=1/η3, and reductant content in exhaust of the third stage can be expressed as ![]() (1/η3)-1. Similarly, the minimum for the second stage can be expressed as M2=1/(3η2). When
(1/η3)-1. Similarly, the minimum for the second stage can be expressed as M2=1/(3η2). When ![]() it indicates that exhaust of the third stage can meet the reductant need of the second stage. Thus, the third stage is key stage; conversely, the second stage is key stage. The relationships between M2 and
it indicates that exhaust of the third stage can meet the reductant need of the second stage. Thus, the third stage is key stage; conversely, the second stage is key stage. The relationships between M2 and ![]() with the change of reducing gas composition at 950 ℃ are shown in Fig. 5.
with the change of reducing gas composition at 950 ℃ are shown in Fig. 5.
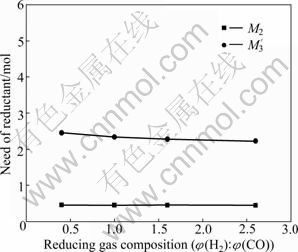
Fig. 5 Relationships between M2 and ![]() with change of reducing gas composition
with change of reducing gas composition
As shown in Fig. 5, with the change of gas composition at 950 ℃, ![]() is always larger than M2. So in this case, the third stage (FeO→Fe) is the key stage in the reduction process and utilization of the reduction process also depends on that of the third stage. Effect of gas composition on the utilization at 950 ℃ is given in Table 6. Changes of gas composition would influence the thermodynamics utilization of the reducing gas. To be specific, the utilization increases with the rise of H2 content.
is always larger than M2. So in this case, the third stage (FeO→Fe) is the key stage in the reduction process and utilization of the reduction process also depends on that of the third stage. Effect of gas composition on the utilization at 950 ℃ is given in Table 6. Changes of gas composition would influence the thermodynamics utilization of the reducing gas. To be specific, the utilization increases with the rise of H2 content.
Table 6 Effect of gas composition on utilization

4 Conclusions
1) Generally, the increase of hydrogen content in reducing gas leads to an obvious increase of reduction rate. When the ratio of φ(H2):φ(CO) increases from 0.4 to 1.6, reduction rate would increase significantly. In the proportion range from 1.6 to 2.6, the reduction rate keeps invariant.
2) Raising temperature can effectively improve the reduction rate. However, with temperature raising to 1 000 ℃, reduction rate is significantly slowed. So, the optimum reduction temperature is about 950 ℃.
3) At 950 ℃, for the most part of reduction process (>90%), interfacial chemical reaction is the rate controlling step. In the last stage (<10%), diffusion of reducing gas becomes the controlling step. With the increase of H2 content, the diffusion control step is significantly shortened.
4) The third stage (FeO→Fe) is the key stage in reduction process at 950 ℃, and the utilization of reducing gas increases with the rise of H2 proportion.
References
[1] FAN Xiao-hui, QIU Guan-zhou, JIANG Tao. Present status and development prospect of iron ore direct reduction [J]. Ironmaking, 2002, 21(3): 53-56. (in Chinese)
[2] HUANG Zhu-cheng. Cold-bonded pellet coal-based direct reduction technology and application [D]. Changsha: School of Minerals Processing and Bioengineering, Central South University, 6-10. (in Chinese)
[3] KRZYSZTOF P, KANCHAN M, HANA L. Effect of gas composition on the kinetics of iron oxide reduction in a hydrogen production process [J]. International Journal of Hydrogen Energy, 2005, 30: 1543-1554.
[4] LI Qiu-ju, HONG Xin. Non-isothermal kinetic model for reduction of ferrous oxide with hydrogen and carbon monoxide [J]. Ironmaking and Steelmaking, 2009, 39(1): 24-28.
[5] LI Qiu-ju, WANG Dao-jing, HONG Xin. Gas reduction mechanism of microscale iron oxide powders [J]. Iron and Steel, 2008, 43(7): 22-26. (in Chinese)
[6] WANG Yu-ming, YUAN Zhang-fu, MATSUURA H. Reduction extraction kinetics of titania and iron from an ilmenite by H2–Ar gas mixtures [J]. ISIJ International, 2009, 49(2): 164-170.
[7] LI Yong-quan, CHEN Hong, ZHOU Yu-sheng. Research and development of coal gas based BL direct reduction process [J]. Special steel, 1999, 20(3): 40-43. (in Chinese)
[8] LI Yong-quan. Kinetic research of pellet reduction in shaft furnace by using two different reducing gases [J]. Technology of Bao Steel, 2000, 18(2): 36-40. (in Chinese)
[9] XU Shi-sen, ZHANG Dong-liang, REN Yong-qiang. Large-scale coal gasification technology [M]. Beijing: Chemical Industry Press, 2006: 102-139. (in Chinese)
[10] HUANG Zhu-cheng, YI Ling-yun, PENG Hu. Experimental research on direct reduction of iron ore pellets by coal gas [C]// The National Annual Meeting of Non-Blast Furnace Iron Making. Beijing: Metallurgical Industry Press, 2010: 174-179. (in Chinese)
[11] HUANG Zhu-cheng, YI Ling-yun, JIANG Tao. Mechanisms of strength decrease in the initial reduction of iron ore oxide pellets [J]. Powder Technology, 2012, 221: 284-291.
[12] WANG You-liu. Ferrous metallurgy (ironmaking) [M]. Beijing: Metallurgical Industry Press, 2005: 87-95. (in Chinese)
[13] USUI T, NOGAMI H, YAGI J. Numerical investigation of simultaneous injection of pulverized coal and natural gas with oxygen enrichment to the blast furnace [J]. ISIJ International, 2002, 42(11): 1203-1211.
[14] PANG Jian-ming, GUO Pei-min, ZHAO Pei. Kinetics of reduction of hematite by H2 using nonisothermal thermogravimetric method [J]. Iron and Steel, 2009, 44(2): 11-15. (in Chinese)
[15] GUO Yu-feng. Study on strengthen the solid-state reduction and comprehensive utilization of vanadiferous tianomagnetite [D]. Changsha: School of Minerals Processing and Bioengineering, Central South University, 35-41. (in Chinese)
[16] FANG Jue. Process and theory of non-blast furnace ironmaking [M]. Beijing: Metallurgical Industry Press, 2002: 57-60. (in Chinese)
(Edited by DENG Lü-xiang)
Foundation item: Project(50725416) supported by National Natural Science Funds for Distinguished Young Scholars of China
Received date: 2011-06-08; Accepted date: 2011-09-22
Corresponding author: HUANG Zhu-cheng, Professor; Tel: +86-731-88830542; E-mail: zchuangcsu@126.com

