
Adsorption properties of Ag(I), Au(III), Pd(II) and Pt(IV) ions on commercial 717 anion-exchange resin
LIU Peng(刘 鹏)1, LIU Guang-feng(刘广峰)1, CHEN Da-lin(陈大林)2,
CHENG Shao-yi(程绍逸)2, TANG Ning(唐 宁)1
1. Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province,
College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China;
2. Jinchuan Group Limited, Jinchang 737104, China
Received 10 August 2009; accepted 15 September 2009
Abstract: The adsorption properties of the four precious metal ions (Ag(Ⅰ), Au(Ⅲ), Pd(Ⅱ) and Pt(Ⅳ)) on the commercial Cl--form 717 strongly basic anion-exchange resin were studied in detail. The effects of the contact time, solution acidity, and concentrations of Cl- and Pb2+ ions on the adsorption properties were studied by the batch method. Then, the column method was conducted under the optimized adsorption conditions (pH=3.0). The effects of the sample loading flow rate and the length-to-diameter ratios of the columns were investigated. The precious metal ions adsorbed could not be eluted completely after the saturated adsorption because the precious metal ions were found to be reduced to their metallic states during the adsorption process. So, it is recommended that the commercial Cl--form 717 strongly basic anion-exchange resin should be decomposed directly to recovery the precious metals after the saturated adsorption.
Key words: adsorption; commercial resin; precious metal ions; optimization; reduction
1 Introduction
Precious metals are used widely in many fields such as catalysts in various chemical processes, electrical and electronic industries, corrosion resistant materials, and jewelry, because of their specific physical and chemical properties. Also, new and emerging applications in energy[1], health[2] and environment[3] are expected for them in the coming years. This gives a compelling reason for developing more efficient and environmental- friendly methods for their extraction and recovery from mineral ores and waste materials (e.g., e-wastes, and industrial effluents).
Many studies have been recently focused on the extraction and separation of precious metals due to both increasing industrial need for these metals and their limited sources. The conventional methods for the removal of the metal ions from water and wastewater include ion exchange, sorption with activated carbon[4-5], silica[6-7], chelating resin[8-9], natural polymer[10-11] and biomass[12], solvent extraction[13], combining agglomeration and adsorption[14] and related processes, involving impregnant resins[15] and liquid membranes[16].
Among the above methods, anion exchange is a highly effective and economical and sometimes irreplaceable method because of the complex-anions of the precious metals[17]. There were many excellent works reporting the adsorption and recovery of precious metal ions with anion-exchange resins, such as Amberlite resins (IRA-93, IRA-68 and IRA-400)[18], TEVA resin[19], Amberlyst A29 and A21[20], Duolite S37[21], and Purolitr A500[22].
The aim of the present work is to study the applicability of 717 resin, a commercial Cl--form strongly basic anion-exchange resin, for the adsorption of the trace-amount Cl--complexes of Ag(Ⅰ), Au(Ⅲ), Pd(Ⅱ) and Pt(Ⅳ) ions from complex wastes. The adsorption conditions were optimized with batch method and column method.
2 Experimental
2.1 Materials
Standard stock solution of Ag(Ⅰ) (500 mg/L) was prepared by dissolving AgNO3 in diluted HNO3. Standard stock solutions of Au (Ⅲ), Pd (Ⅱ) and Pt (Ⅳ) (1 g/L) were prepared by dissolving spectrometric pure HAuCl4?H2O, PdCl2 and (NH4)2PtCl6 in 1 mol/L HCl, respectively. Standard solutions were prepared weekly by dilution of the standard stock solutions with 0.5 mol/L HCl, respectively. All the other reagents used were of analytical-reagent grade. Distilled water was used throughout.
The Cl--form 717 strongly basic anion-exchange resin was a commercial resin (particle size 0.5 mm, water content 42%-48%, exchange capacity 3.6 mmol/g wet resin) and used without any treatment.
2.2 Batch method
Wet resin 0.20 g was added into 25 mL aqueous solution containing 0.40 mg/mL of the four precious metal ions in HCl medium. Then, the mixture was shaken mechanically for 1-30 min at room temperature. After a certain time, the phases were separated by filtration in order to determine the concentrations of the precious metal ions in the solution.
The adsorption rate (RA) of the precious metal ions is calculated from the followed equation:
RA/%=(c0-cw)/c0×100 (1)
where cw and c0 are the concentrations of the precious metal ions in the aqueous solutions after and before adsorption, respectively.
2.3 Column method
Three glass columns were packed with certain amounts of the wet 717 resin and used for the column adsorption (Table 1). The resin was packed into the columns after being activated in 0.5 mol/L HCl aqueous medium for 24 h.
Table 1 Three glass columns used
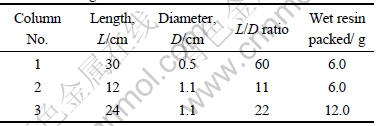
The mixture solution containing the four precious metal ions was passed through the columns with a certain sample loading flow rate controlled by a peristaltic pump. The solution effused was collected for the ICP-AES detection. The adsorption rate was calculated with the Eq.(1).
2.4 Instrumentation
An ICP/6500 inductively coupled plasma atomic emission spectrometer (ICP-AES, Perkin-Elmer) was used for the detection of the concentrations of the precious metal ions. The analysis conditions were: forward power 1 100 W, viewing height 15 mm, argon plasma gas flow rate 15 L/min, argon nebulizer gas flow rate 1.0 L/min, argon intermediate gas flow rate 0.7 L/min, and wavelengths Ag 328.068 nm, Au 242.795 nm, Pd 324.270 nm, Pt 214.423 nm.
The surface morphologies of resins were observed using scanning electron microscope (SEM) (XL-20, Philips Corporation, the Netherlands), operating at 25 kV.
3 Results and discussion
3.1 Batch method
The effect of the contact time on the adsorption rates of the four precious metal ions in pH 2.0 HCl medium is shown in Fig.1. It was found that the adsorption of Ag(Ⅰ) ions was the fastest in the competitive adsorption. Its adsorption rate reached more than 95% within only 1 min. That of Au(Ⅲ) needed 2 min. However, the adsorption rates of Pd(Ⅱ) and Pt(Ⅳ) reached 90% in more than 5 and 30 min, respectively.
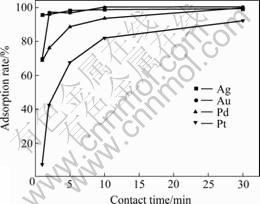
Fig.1 Effect of contact time on adsorption rates
So, the contact time was selected as 30 min in the batch method. The effect of the sample acidity on the adsorption rates of the four precious metal ions for 30 min is shown in Fig.2. More than 98% of the Ag(Ⅰ) and Au(Ⅲ) ions were adsorbed in the full acidity range studied. The adsorption rate of Pd(Ⅱ) and Pt(Ⅳ) reached their maximum (98.6% for Pd(Ⅱ) and 86.8% for Pt(Ⅳ)) at pH value of 3.0. So, the optimal adsorption acidity was selected as pH 3.0.
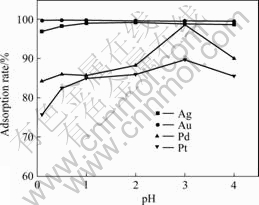
Fig.2 Effect of sample acidity on adsorption rates
It is well known that the precious metal ions are adsorbed by the anion-exchange resin in the complex form with Cl- ions[23]. So, the adsorption rate of the precious metal ions was affected markedly by the Cl- ion concentration in the sample solutions. The adsorption rate of Ag(Ⅰ), Au(Ⅲ) and Pd(Ⅱ) ions reached their maximum with the NaCl content of 0.50% (Fig.3); that of Pt(Ⅳ) remained 91.5%-92.0% in the range of the NaCl content of 0.20%-1.00%. So, the 0.50% NaCl was added in the sample solutions to provide the optimal Cl- ions concentration in the further experiments.
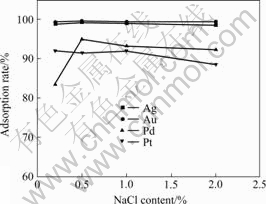
Fig.3 Effect of NaCl content on adsorption rates
Among the common co-existing heavy metal ions, Pb2+ ion mainly exhibits the Cl--complex forms in the HCl medium. The Cl- complex of Pb2+ ion could be adsorbed onto the anion-exchange resin, so it maybe interferes the adsorption of the precious metal ions. The effect of the concentration ratio of Pb2+ ion (cPb) to the total precious metal ions (cPM) on the adsorption rate of the precious metal ions is shown in Fig.4. It was found that the adsorption rate of Ag(Ⅰ) and Au(Ⅲ) ions decreased slightly with increasing the concentration ratio. The adsorption rate of Pd(Ⅱ) and Pt(Ⅳ) ions were more than 90% in the studied concentration ratio range. This showed that the Cl--form 717 strongly basic anion- exchange resin had good selectivity for the precious metal ions.
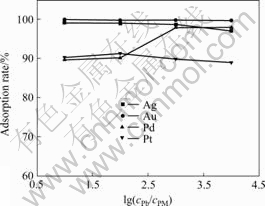
Fig.4 Effect of Pb2+ ion concentration ratio on adsorption rates
3.2 Column method
In the adsorption experiments using the batch method, the best efficiencies were achieved in no less than 30 min. So, the batch method is too inefficient to be conducted in the practical applications.
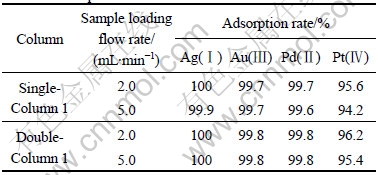
In the column method, three glass columns with different length-to-diameter ratios (L/D ratio) and volume were used (Table 1). 100 mL of the mixture sample solution containing 5.0 mg/mL each of the four precious metal ions was passed through the Column 1 with a certain sample loading flow rate. 25 mL of the solution effused was collected for the ICP-AES detection to calculate the adsorption rate after 40 mL of the solution passed through. 25 mL of 0.5 mol/L HCl solution was used to wash the column after the sample solution passed through the column completely. The results are given in Table 2. It was found that the best adsorption was achieved with the lower sample loading flow rate studied (0.70 mL/min). The adsorption rate of the four precious metal ions was higher than 95%.
Table 2 Adsorption rates with different columns and different sample loading flow rates

As for Column 2 with different L/D ratio and same packed volume (5.89 mL) as Column 1 and Column 3 with different L/D ratio and double packed volume as Column 1, the higher adsorption rates were achieved with the same sample loading flow rates (0.70 mL/min), compared with those of Column 1. This indicated that the lower L/D ratio with the same packed volume and the longer contact time favored the adsorption of the precious metal ions.
However, the low sample loading flow rates (0.70 mL/min) equivalent to about 12% column packed volume per minute is so slow for the practice application. Therefore, the adsorption experiments were conducted with the double-column in series of Column 1 with the higher sample loading flow rates. The results are summarized in Table 3. It could be concluded that the higher sample loading flow rates could be used for the practice application with double-column in series technique.
Table 3 Adsorption rates with double-column in series
3.3 Saturated adsorption
The saturated adsorptions of the four precious metal ions were conducted by addition of 0.1 g wet resin into a industrial waste water containing higher concentrations of the four precious metal ions. The mixture was shaken mechanically for 24 h. The resin became brown and black later during the adsorption (Fig.5). The resin after saturated adsorption was decomposed and dissolved with aqua regia for the ICP-AES analysis after being filtered and washed thoroughly with water. The four precious metal ions adsorbed on the resin were found to be 6.25 g (0.058 mol) for Ag(Ⅰ), 746.5 g (3.79 mol) for Au(Ⅲ), 701.5 g (6.59 mol) for Pd(Ⅱ) and 3.44 mg (17.65 mol) for Pt(Ⅳ) per 0.1 g wet resin, respectively. If the four precious metal ions were calculated as the forms of AgCl2-, AuCl4-, PdCl42- and PtCl62-, the total anion exchanged content was found to be 5.2 mmol/g wet resin. It was much higher than the exchange capacity of 3.6 mmol/g wet resin given by the manufacturer.
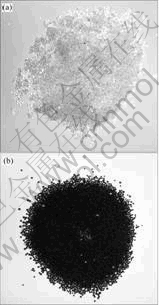
Fig.5 Photos of resin before (a) and after (b) saturated adsorption
The surface of the fresh resin and the resin after saturated adsorption were compared by the SEM technique (Fig.6). Amounts of the particles were found on the resin after saturated adsorption compared with the surfaces of the fresh resin. This might due to the reduction of the precious metal ions.
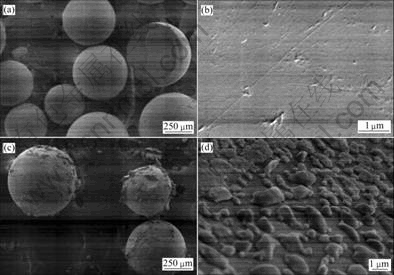
Fig.6 SEM images of resin before ((a) and (b)) and after ((c) and (d)) saturated adsorption
In order to validate the reduction of the precious metal ions adsorbed, the resin after saturated adsorption was eluted with 0.5% TU+0.5 mol/L HCl as eluent with eluting flow rate of 0.70 mL/min for 72 h[23]. Any of the precious metal ions could not be detected after the elution of 48 h. But the resin showed red-brown color, not the bright yellow color of the fresh resin. This showed that the precious metal ions could not be eluted completely and parts of the precious metal ions had been reduced to their metallic states.
4 Conclusions
1) The commercial Cl--form 717 strongly basic anion-exchange resin had excellent adsorption properties towards the four precious metal ions (Ag(Ⅰ), Au(Ⅲ), Pd(Ⅱ) and Pt(Ⅳ)).
2) The lower L/D ratio with the same packed volume and the longer contact time favored the adsorption of the precious metal ions in the column method.
3) The precious metal ions could be reduced to their metallic states after saturated adsorption on the commercial Cl--form 717 strongly basic anion-exchange resin.
References
[1] Farrauto R, LIU Y, Ruettinger W, Ilinich O, Shore L, Giroux T. Precious metal catalysts supported on ceramic and metal monolithic structures for the hydrogen economy [J]. Catalysis Reviews-Science and Engineering, 2007, 49(2): 141-196.
[2] HUANG X H, EL-SAYED I H, QIAN W, EL-SAYED M A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods [J]. Journal of the American Chemical Society, 2006, 128(6): 2115-2120.
[3] GELIN P, PRIMET M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review [J]. Applied Catalysis B: Environmental, 2002, 39(1): 1-37.
[4] JIA Y F, DEMOPOULOS G P. Adsorption of silver onto activated carbon from acidic media: Nitrate and sulfate media [J]. Industrial & Engineering Chemistry Research, 2003, 42(1): 72-79.
[5] COX M, PICHUGIN A A, EL-SHAFEY E I, APPLETON Q. Sorption of precious metals onto chemically prepared carbon from flax shive [J]. Hydrometallurgy, 2005, 78(1/2): 137-144.
[6] KANG T, PARK Y, YI J. Highly selective adsorption of Pt2+ and Pd2+ using thiol-functionalized mesoporous silica [J]. Industrial & Engineering Chemistry Research, 2004, 43(6): 1478-1484.
[7] KRAMER J, DHLADHLA N E, KOCH K R. Guanidinium functionalised silica-based anion exchangers significantly improve the selectivity of platinum group metal recovery from process solutions [J]. Separation and Purification Technology, 2006, 49(2): 181-185.
[8] SANCHEZ J M, HIDALGO M, SALVADO V. Selective adsorption of gold(Ⅲ) and palladium(Ⅱ) on new phosphine sulphide-type chelating polymers bearing different spacer arms: Equilibrium and kinetic characterization [J]. Reactive and Functional Polymers, 2001, 46(3): 283-291.
[9] DONIA A M, ATIA A A, ELWAKEEL K Z. Gold(III) recovery using synthetic chelating resins with amine, thio and amine/mercaptan functionalities [J]. Separation and Purification Technology, 2005, 42(2): 111-116.
[10] FUJIWARA K, RAMESH A, MAKI T, HASEGAWA H, UEDA K. Adsorption of platinum (Ⅳ), palladium (Ⅱ) and gold (Ⅲ) from aqueous solutions onto l-lysine modified crosslinked chitosan resin [J]. Journal of Hazardous Materials, 2007, 146(1/2): 39-50.
[11] KANAI Y, OSHIMA T, BABA Y. Synthesis of highly porous chitosan microspheres anchored with 1,2-ethylenedisulfide moiety for the recovery of precious metal ions [J]. Industrial & Engineering Chemistry Research, 2008, 47(9): 3114-3120.
[12] MACK C, WILHELMI B, DUNCAN J R, BURGESS J E. Biosorption of precious metals [J]. Biotechnology Advances, 2007, 25(1): 264-271.
[13] SHEN Y F, XUE W Y. Recovery palladium, gold and platinum from hydrochloric acid solution using 2-hydroxy-4-sec-octanoyl diphenyl-ketoxime [J]. Separation and Purification Technology, 2007, 56(3): 278-283.
[14] ZUO Hua-sheng, CHU Zhu-qiang, LIN Gang. A novel recovery technology of trace precious metals from waste water by combining agglomeration and adsorption [J]. Trans Nonferrous Met Soc China, 2007, 17(4): 858-863.
[15] Saitoh T, Nakane F, Hiraide M. Preparation of trioctylamine-impregnated polystyrene-divinylbenzene porous resins for the collection of precious metals from water [J]. Reactive and Functional Polymers, 2007, 67(3): 247-252.
[16] Fontas C, Antico E, Vocanson F, Lamartine R, Seta P. Efficient thiacalix[4]arenes for the extraction and separation of Au(Ⅲ), Pd(Ⅱ) and Pt(Ⅳ) metal ions from acidic media incorporated in membranes and solid phases [J]. Separation and Purification Technology, 2007, 54(3): 322-328.
[17] Bernardis F L, Grant R A, Sherrington D C. A review of methods of separation of the platinum-group metals through their chloro-complexes [J]. Reactive and Functional Polymers, 2005, 65(3): 205-217.
[18] Gaita R, AL-BAZI S J. An ion-exchange method for selective separation of palladium, platinum and rhodium from solutions obtained by leaching automobile catalytic converters [J]. Talanta, 1995, 42(2): 249-255.
[19] Makishima A, Nakanishi M, Nanamura E. A group separation method for ruthenium, palladium, rhenium, osmium, iridium, and platinum using their bromocomplexes and an anion exchange resin [J]. Analytical Chemistry, 2001, 73(21): 5240-5246.
[20] Hubicki Z, Wolowicz A, Leszczynska M. Studies of removal of palladium(II) ions from chloride solutions on weakly and strongly basic anion exchangers [J]. Journal of Hazardous Materials, 2008, 159(2/3): 280-286.
[21] HUBICKI Z, WOJCIK G. Studies of removal of platinum(Ⅳ) ions microquantities from the model solutions of aluminium, copper, iron, nickel and zinc chloride macroquantities on the anion exchanger Duolite S37 [J]. Journal of Hazardous Materials, 2006, 136(3): 770-775.
[22] Jayasinghe N S, Lucien F P, Tran T. Ion-exchange equilibria for [Au(CN)2]-/Cl- and Au(CN)2-/SCN- on purolite A500 in mixed solvents at 303 K [J]. Industrial & Engineering Chemistry Research, 2005, 44(19): 7496-7504.
[23] LIU P, PU Q S, HU Z D, SU Z X. On-line preconcentration and separation of platinum using thiourea modified silica gel with microwave assisted desorption for FAAS determination [J]. Analyst, 2000, 125(6): 1205-1209.
Corresponding author: LIU Peng; Tel: +86-931-8912516; Fax: +86-931-8912582; E-mail: pliu@lzu.edu.cn
DOI: 10.1016/S1003-6326(09)60061-3
(Edited by YANG Bing)