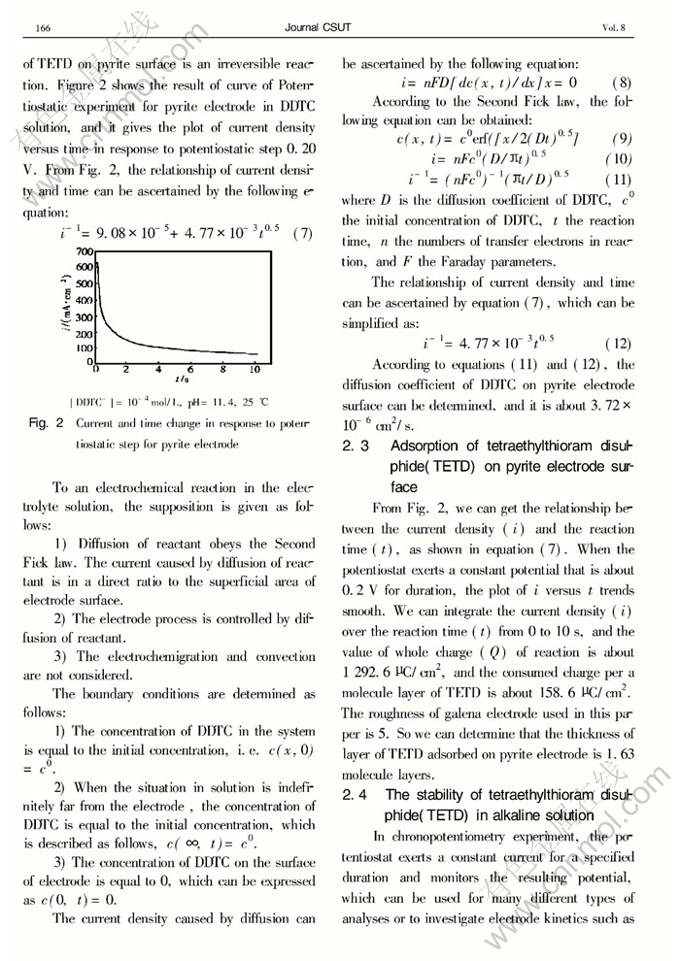
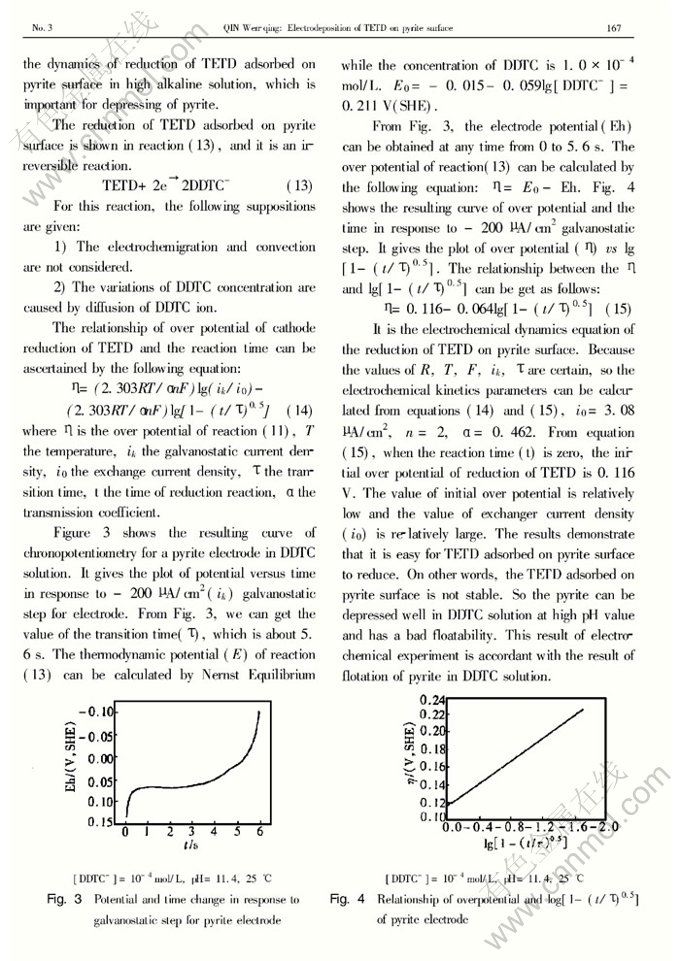
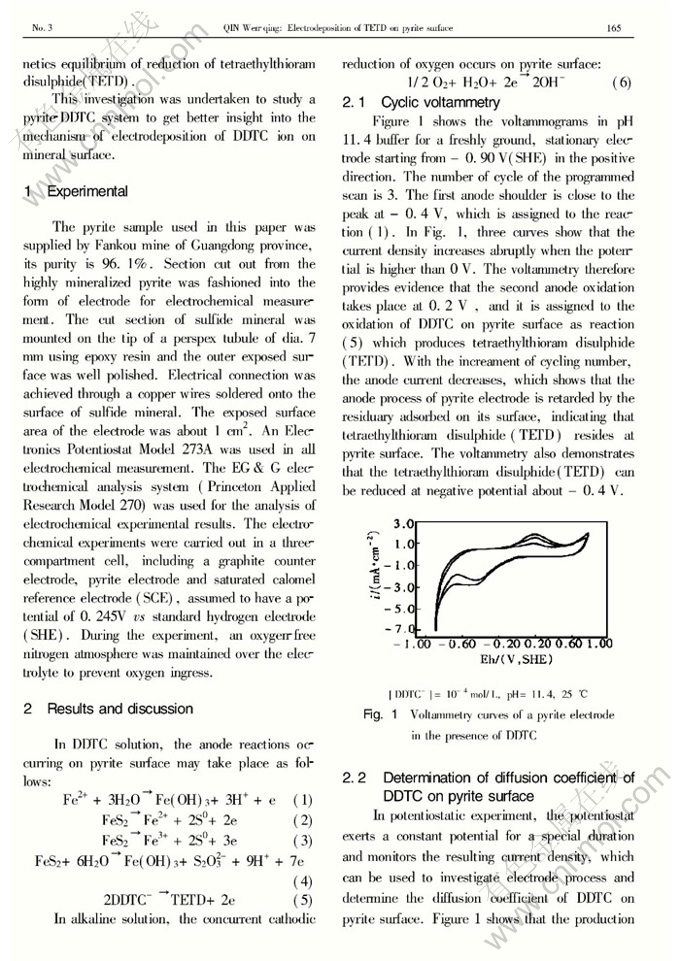
Dynamics of electrodeposition of tetraethylthioram disulphide(TETD) on pyrite surface
来源期刊:中南大学学报(英文版)2001年第3期
论文作者:QIN Wen-qing QIU Guan-zhou HU Yue-hua XU Jing
文章页码:164 - 168
Key words:electrode process; pyrite; electrochemistry of flotation
Abstract: The electrode process of pyrite in diethyldithiocarbamate (DDTC) solution pH 11.4 was investigated by using cyclic voltammetry, potentiostatic and chronopotentiometry. Tetraethylthioram disulphide (TETD) was electrodeposited on pyrite electrode surface as the electrode potential is higher than 0.2 V. The relationship of the current density caused by diffusion and reaction time can be ascertained asi=1/(9.08×10-5+4.77×10-3t0.5), and the diffusion coefficient of DDTC on pyrite surface is about 3.72×10-6cm2/s. At pH11.4, the thickness ofTETD adsorbed on pyrite surface is about 1.63 molecule layer. The electrochemical dynamics equation of the reduction of TETD on pyrite surface is given asη=0.116-0.064log[1-(t/τ)0.5]. The kinetic parameters were determined as follows: the exchange current density (i0) is 3.08μA/cm2; the transmission coefficient(α) is 0.462.

.