基于地球化学的水热还原矿化稳定砷的技术思路
来源期刊:中国有色金属学报2020年第4期
论文作者:胡斌 杨天足 刘伟锋 张杜超 陈霖
文章页码:847 - 858
关键词:砷;三硫化二砷;稳定化;水热;矿化
Key words:arsenic; arsenic trisulfide; stabilization; hydrothermal; mineralization
摘 要:砷作为有色金属矿物的共伴生元素,在有色金属冶炼过程中以含砷“三废”形式大量产出。由于砷具有强致癌性及毒性,导致砷的安全处置问题严重困扰着有色金属冶炼企业。本文通过阐述含砷废水中砷的两种稳定化工艺的研究进展,对比了现有稳定化工艺的优缺点,结合药剂稳定化和矿物稳定化的优点,借鉴砷元素在地球化学中的成矿规律,提出了硫化沉砷-水热还原矿化稳定砷的技术思路。首先采用硫化法脱除含砷废水中的砷,砷的沉淀率高达99.65%,硫化沉淀物在TCLP毒性检测中砷的浓度达到212.9 mg/L。然后采用As-S系一元水热还原矿化法和As-Fe-S系二元水热还原矿化方法稳定砷,稳定化产物分别为雌黄和雌黄-铁硫系(黄铁矿、硫化亚铁)混合物,在TCLP毒性检测中砷的浸出浓度分别为3.86 mg/L和2.65 mg/L。水热还原矿化工艺实现了含砷废水中砷的脱除及稳定化的目的,为水溶液中砷的脱除和稳定化提供了新的思路。
Abstract: Arsenic is one of paragenetic and associated elements in non-ferrous minerals, which is enriched in “the three wastes” containing arsenic during the smelting of nonferrous metal processes. The safety disposal of wastewater containing arsenic has troubled nonferrous metals smelting enterprises due to the strong carcinogenicity and toxicity of arsenic. This paper firstly introduced the sources and harm of wastewater arsenic-containing. And then the stabilization technologies including chemical stabilization and mineral stabilization were reviewed. By comparing the advantages and disadvantages of stabilization technologies for treating wastewater containing arsenic, and referring to the mineralization rule of arsenic in geochemistry, a sulfidization-hydrothermal reduction mineralization process of arsenic was proposed. The results show that, firstly, 99.65% arsenic in wastewater containing arsenic was precipitated in the form of amorphous As2S3 by using Na2S. Then the leachate concentration of arsenic of amorphous As2S3 in TCLP test is 212.97 mg/L. Next, the hydrothermal reduction mineralization process in As-S unary system and hydrothermal reduction mineralization process in As-Fe-S binary system are adopted to transform amorphous As2S3 to orpiment and orpiment and iron-sulfur system (pyrite, ferrous sulfide) mixture separately. And the As leachate concentration of these corresponding hydrothermal slags in the TCLP test is reduced to 3.86 mg/L, 2.65 mg/L separately. A satisfied result of removal and stabilization of arsenic from wastewater are achieved by the novel processes, which provides a promising way to remove and stabilize arsenic from high arsenic containing wastewater.
DOI: 10.11817/j.ysxb.1004.0609.2020-35741
胡 斌,杨天足,刘伟锋,张杜超,陈 霖
(中南大学 冶金与环境学院,长沙 410083)
摘 要:砷作为有色金属矿物的共伴生元素,在有色金属冶炼过程中以含砷“三废”形式大量产出。由于砷具有强致癌性及毒性,导致砷的安全处置问题严重困扰着有色金属冶炼企业。本文通过阐述含砷废水中砷的两种稳定化工艺的研究进展,对比了现有稳定化工艺的优缺点,结合药剂稳定化和矿物稳定化的优点,借鉴砷元素在地球化学中的成矿规律,提出了硫化沉砷-水热还原矿化稳定砷的技术思路。首先采用硫化法脱除含砷废水中的砷,砷的沉淀率高达99.65%,硫化沉淀物在TCLP毒性检测中砷的浓度达到212.9 mg/L。然后采用As-S系一元水热还原矿化法和As-Fe-S系二元水热还原矿化方法稳定砷,稳定化产物分别为雌黄和雌黄-铁硫系(黄铁矿、硫化亚铁)混合物,在TCLP毒性检测中砷的浸出浓度分别为3.86 mg/L和2.65 mg/L。水热还原矿化工艺实现了含砷废水中砷的脱除及稳定化的目的,为水溶液中砷的脱除和稳定化提供了新的思路。
关键词:砷;三硫化二砷;稳定化;水热;矿化
文章编号:1004-0609(2020)-04-0847-11 中图分类号:X703.1;X705;X758 文献标志码:A
砷是一种广泛存在于自然界的非金属元素,其单质和化合物主要用于合金冶炼、农药医药、颜料等工业中。砷在自然界的矿物多达300多种,其中最常见的含砷矿物有毒砂(FeAsS)、雌黄(As2S3)、雄黄(AsS)等[1]。由于砷常与铜、铅、锌、金等金属的矿物共伴生,因此在这些矿物的开采、冶炼过程中,砷便以砷酸盐、硫化物、氧化物等形式进入了冶炼厂的废气、废水及废渣中。据统计,我国有色金属冶炼行业每年会产生3200万t工业危废[2],以铜冶炼为例,2017年我国仅在铜冶炼过程产生的含砷烟尘就高达44万t[3]。其中有色冶炼废气经收尘、洗涤净化后用于制备硫酸,而在洗涤过程中,大量的砷和部分SO2溶于洗涤水形成了酸性含砷废水。由于具有产量大、酸度高、砷浓度高等特点,含砷废水已成为我国砷的主要污染源之一。由于砷是一种强致癌性的元素,且砷的化合物几乎都具有毒性大、迁移性强的特点,若任由砷进入生态系统,将引起严重的环境问题(水污染、土壤污染等),进而造成植物枯死,人类及动物中毒的后果[4]。因此,在日益严峻的环保形势下,如何资源化利用或治理含砷废水已成为冶炼企业亟待解决的难题。
目前,含砷废水的处理方式主要分为资源化和稳定化。由于来自冶炼厂的含砷废水一般都含有相当数量的有价金属,如铜、铅、锌等,常作为二次资源被选择性回收,同时对砷的资源化也做了研究[5-7]。但由于砷的强毒性,导致含砷产品在工、农业中需求极其有限,回收砷的成本也很高,因此砷的资源化之路仍然任重道远。但是为了解决含砷废水储存导致的环境安全和环境污染问题,需将废水中的砷转化成环境稳定的含砷物质,防止二次污染,因此科研工作者提出了砷的稳定化工艺。
本文在对比含砷废水中砷的稳定化工艺特点的基础上,针对稳定化产物中含砷量低、增容比高的问题,借鉴砷在地球化学中的行为规律[8],提出了硫化沉砷-水热还原矿化工艺实现砷的稳定化。水热还原矿化工艺按照目标产物不同,分为As-S系一元水热还原矿化工艺和As-Fe-S系二元水热矿化工艺,期望以雌黄/雄黄或砷黄铁矿的形式稳定砷,为含砷危废的“减量化”提供了新的研究思路。
1 含砷废水中砷的稳定化研究现状
1.1 概述
含砷废水主要来自有色金属的火法冶炼过程,在火法冶炼过程中,大部分易挥发元素如铅、锌、砷、锑、硫等不同程度的挥发进入烟气,再经收尘、净化等工序将这些元素脱除,同时得到了含砷烟尘及酸性含砷废水[9]。含砷废水中砷的稳定化工艺主要有药剂稳定化和矿物稳定化。含砷废水的稳定化工艺的对比见表1。
1.2 药剂稳定化
药剂稳定化是通过向含砷废水中加入化学试剂或其他的外加试剂,将含砷废水中不稳定的砷转化成性质稳定的含砷化合物,达到降低砷的毒性和溶解迁移性的目的[10]。该技术主要用来处理重金属废物,典型的药剂稳定化技术包括:pH值控制技术、氧化/还原电势控制技术及沉淀技术[11]。其中应用最广的为化学沉淀法,在实际应用中化学沉淀法也利用了pH值和氧化还原电势控制的原理,如钙盐法可提高pH并脱除重金属,将三价砷氧化为五价砷有助于砷的沉淀脱除或吸附等。本文主要介绍钙盐沉淀法、铁盐沉淀法、硫化沉淀法及协同稳定化。
表1 含砷废水的稳定化工艺对比
Table 1 Comparison of stabilization and solidification technologies of three wastes containing arsenic
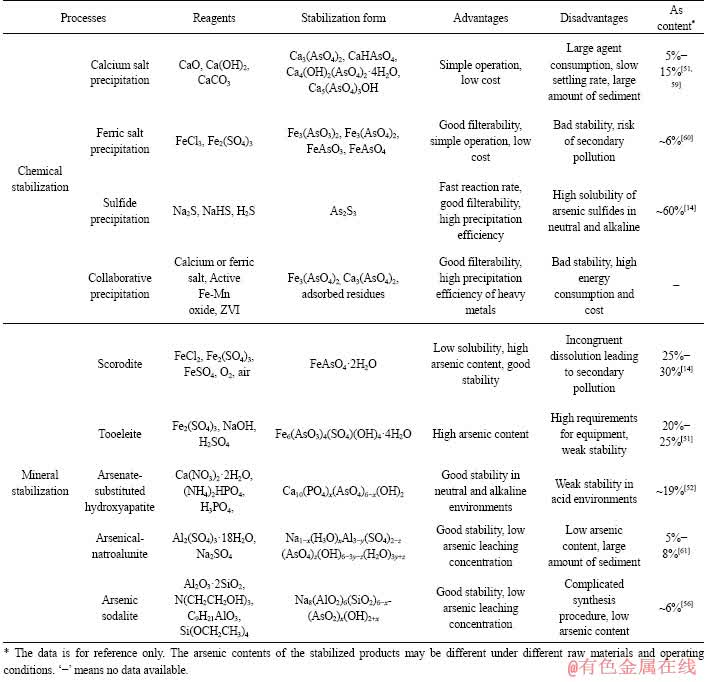
1.2.1 钙盐沉淀法
钙盐沉淀法主要利用砷酸根和亚砷酸根与钙离子可生成砷酸钙与亚砷酸钙沉淀的原理,达到将水溶砷转化为更稳定的砷钙渣的目的。常用的钙盐有氧化钙、氢氧化钙、电石渣等[12-13]。此外,考虑到三价砷的毒性远强于五价砷,且砷酸盐沉淀的溶度积也更小,故而常将三价砷氧化为五价后再处理[14]。对于碱性含砷废水,多采用石灰沉淀法稳定化砷,而且砷钙渣的沉淀形式与初始pH有关[15]。对于酸性废水,采用钙盐沉淀砷的同时,还将促进其他重金属离子的水解沉淀,达到净化水质的目的,但同时会消耗大量的钙盐,生成大量成分复杂的石膏渣,且砷钙渣也易于空气中的二氧化碳反应导致砷的复溶[16]。因此有研究表明在800 ℃下直接煅烧砷钙渣,可使其转化为稳定性较好的砷钙渣,砷主要以晶态Ca5(AsO4)3OH存在[15]。也有选用SiO2、Al2O3、MgO和CaO与砷渣混合后在1000 ℃下煅烧稳定砷的研究,结果证明MgO、CaO对砷有较强的固定作用,特别是CaO可显著抑制烧结体中砷的浸出,起到了固砷的作用[17]。
1.2.2 铁盐沉淀法
铁盐沉淀法主要是基于砷酸根、亚砷酸根可与铁离子生成难溶沉淀砷酸铁、亚砷酸铁的原理脱砷、稳定砷。且当调高反应pH时会有大量氢氧化铁胶体形成,可与废水中的砷及重金属离子发生吸附共沉淀,从而得到较高的除砷率[18-19]。铁盐沉淀法由于沉淀工艺条件的不同,得到的砷铁渣有多种物相,如碱式砷酸铁、砷酸铁等,以及氢氧化铁胶体吸附的砷,这种沉淀渣的结晶性及稳定性欠佳,在水中易溶解向环境释放砷[20]。因此,铁盐沉砷的条件控制需严格控制。
1.2.3 硫化沉淀法
硫化沉淀法基于砷离子与硫离子极易反应生成难溶硫化物沉淀的原理,达到脱除砷和重金属的目的[21-22]。常用的硫化剂主要有Na2S、NaHS、H2S等。近年有关生物硫化法的研究也相继涌现,这类硫化法主要是利用细菌在废水中分解产生硫化氢,再与废水中的重金属离子发生硫化沉淀反应脱除重金属的方法[23-25]。由于硫化沉淀法具有反应速度快、产物稳定、过滤性能好等优点,在重金属废水的治理中应用广泛[26]。但是硫化砷渣的稳定性不佳,易受空气中的氧和环境中的细菌的侵蚀溶解,造成二次污染。
1.2.4 协同稳定化
为了达到更好的脱砷、稳定砷的效果,常多种方法联用协同稳定砷。如利用铁盐-絮凝工艺从废水中脱砷稳定砷[27],利用铁盐-钙盐复配药剂稳定化含砷废水和污泥[28-29]以及利用铁-锰二元氧化物氧化并吸附三价砷[30]等。基于铁系化合物对砷的强吸附作用[31-32],亦有利用亚铁盐(FeCl2)稳定砷矿渣的研究[33]。针对含砷渣如砷酸钙、砷酸铁及砷吸附渣等的稳定性欠佳,易产生二次污染的问题,有学者提出利用机械活化技术制备的高活性材料(改性零价铁(ZVI))处理铁砷渣[34-35],也实现了稳定砷的目的。
利用外加试剂稳定砷,更多的是利用稳定剂与可溶砷生成沉淀的性质或对砷的强吸附能力,但是稳定后的含砷渣受环境因素如pH、温度、环境的氧化还原电位等的影响较大,因此其稳定性及环境适应性欠佳,若要长期堆存,仍需强化其稳定性。
1.3 矿物稳定化
在含砷废水中砷的脱除及稳定化过程中,为了缩短除砷、稳定砷的流程,并达到稳定砷的目的,提出了以矿物形式除砷、稳定砷的方法即矿物稳定化,研究较多的如臭葱石法、图水羟砷铁矿(Tooeleite)法等。
1.3.1 臭葱石法
天然臭葱石(Scorodite,化学式为FeAsO4·2H2O)于1817年首先在德国被发现,是硫化物矿床氧化带中最常见的砷酸盐矿物,常见于高温或中温热液矿床中。臭葱石的溶度积小,含砷量高(32%),易分离,浸出毒性小,是很好的固砷载体[36-37]。以臭葱石形式除砷并固砷的研究由来已久,人工合成臭葱石的方法有常压合成法[38-39]、水热合成法[40-41]及改进常压法[42-44]等,刘志宏等[45]研究了不同工艺制备的臭葱石的稳定性差异,但是有研究表明在长期堆存时,臭葱石在中性或近中性时会缓慢发生不一致溶解,释放出一定量的砷,因此仍需控制好堆存的条件[46-47]。
1.3.2 图水羟砷铁法
图水羟砷铁矿(Tooeleite,化学式为Fe6(AsO3)4- (SO4)(OH)4·4H2O)于1991年在美国犹他州矿区被发现,由于图水羟砷铁矿在强酸性时仍能稳定存在,因此被广泛研究并应用于砷的脱除及稳定化[48-50]。CHAI等[51]研究了从高砷废水中以图水羟砷铁矿形式除砷的工艺条件,结果表明在最佳条件下,砷的脱除率可达99%,且该含砷渣在pH<4.5时,物相主要以图水羟砷铁矿为主,TCLP试验表明砷的浸出浓度也远低于较高pH下得到的含砷渣,但仍高于国家标准关于砷浸出毒性的限值,尚需进一步提高其稳定性才
能安全堆存。
1.3.3 其他矿物稳定法
此外,科研工作者也发现了一些稳定性较好的矿物形式,在一定条件下,使砷氧阴离子集团替代该矿物中某些元素集团达到稳定砷的目的,也取得了不错的效果。朱琳[52]基于羟基磷灰石(Ca10(PO4)6(OH)2)的结构稳定性,将与P同族的As引入羟基磷灰石中,以 取代部分
取代部分 ,采用水热法使As嵌入到羟基磷灰石晶格中,形成较为稳定的砷羟基磷灰石固溶体,达到固砷的目的。经浸出试验检测,表明砷的浸出浓度受pH值影响很大,在酸性溶液中砷的浸出浓度最高,在中性溶液中砷的浸出浓度最低,证明固溶体在中性环境中更为稳定。
,采用水热法使As嵌入到羟基磷灰石晶格中,形成较为稳定的砷羟基磷灰石固溶体,达到固砷的目的。经浸出试验检测,表明砷的浸出浓度受pH值影响很大,在酸性溶液中砷的浸出浓度最高,在中性溶液中砷的浸出浓度最低,证明固溶体在中性环境中更为稳定。
VINALS等[53]以铜冶炼厂烟气净化砷钙渣为原料,经硫酸及臭氧预处理后,加入Al2(SO4)3·18H2O、Na2SO4水热合成了砷钠明矾石,浸出毒性检测结果显示,在pH为4~5并浸出6个月时,砷的浸出浓度低于0.1 mg/L,表明砷钠明矾石适用于长期堆存。SUNYER等[54-55]采用水热法对砷替代钠明矾石进行了研究,并对其稳定性进行了毒性检测,结果表明,砷钠明矾石成分单一时,其稳定性可满足长期堆存的要求。陶志超[56]以偏高岭土(Al2O3·2SiO2)、纯试剂十二水合砷酸钠(Na3AsO4·12H2O)、乙醇胺(N(CH2CH2OH)3)、异丙醇铝(C9H21AlO3)和正硅酸乙醋(Si(OCH2CH3)4)为原料,水热合成了砷方钠石,并对其进行了表征及稳定性检测,结果表明砷方钠石具有较好的稳定性。但是在最佳合成条件下,砷的沉淀率仅为14.2%,作为稳定化产物,其砷含量偏低,会导致体量的增大。
矿物稳定砷的工艺中,臭葱石矿化工艺成熟度较高,但是臭葱石在长期堆存中易发生不一致溶解,需控制好堆存的条件。图水羟砷铁矿在较高pH值下的稳定性不及较低pH值时,砷钠明矾石及砷方钠石水热矿化产物虽然稳定性较好,但是合成成本较高。三种矿物中砷的含量均不高,这将导致砷稳定化产物体量及所需堆存空间增大。因此,在保证稳定性的前提下,有必要对提高稳定化产物含砷量方面展开研究。
2 含砷废水中砷的水热稳定化新工艺
2005年4月1日起修订的《中华人民共和国合同法固体废物污染环境防治法》规定了固体废物污染防治的“减量化、无害化、资源化”的三化原则,因此在减少固体废物的产生量和危害性同时,应充分回收利用固体废物中的有价成分,最终实现固体废物的资源化,促进清洁生产和循环经济的发展。根据表1中对含砷废水的稳定化工艺的对比,可知经过处理后的稳定化产物中砷含量最高的为硫化沉淀法得到的硫化砷渣,而其他工艺处理后的稳定化产物中砷的含量均不高,这将导致最终稳定化产物的体积急剧增大,不利于含砷危废的减量化。此外,根据地球化学中关于内生成矿作用的介绍,砷在自然界的常见的存在形式主要是雌黄、雄黄及毒砂,而这三种矿物均为热液成矿作用的产物,其中雌黄、雄黄为中低温热液矿物,毒砂为中高温热液矿物,因此采用水热工艺模拟三种矿物的成矿条件,或能以自然矿物的形式实现砷的稳定化[57]。
综上所述,本文提出用硫化沉淀法对高砷废水中的砷进行脱除,再采用水热还原矿化工艺,在高温高压下使促雌黄/雄黄或毒砂矿物的形成,期望能达到提高As2S3稳定性的目的。工艺流程见图1所示。
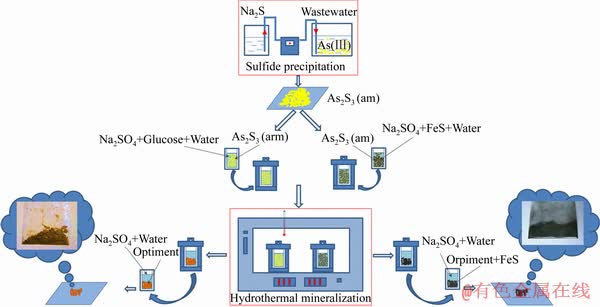
图1 硫化沉砷-一元水热还原矿化、二元水热还原矿化稳定砷的工艺流程图
Fig. 1 Flow chart of removal and stabilization of arsenic by unary and binary hydrothermal reduction and mineralization process
2.1 实验原料
本工艺中研究对象为某冶炼企业的含砷污酸经控制电位选择性脱铜后的含砷废水[58],透明无色,pH=0.6,含砷废水的化学成分见表2。
2.2 硫化沉砷
硫化法主要是利用硫化物溶解度小,反应迅速等特点,达到快速脱除废水中重金属离子的目的。常见的硫化物如As2S3,FeS的溶度积常数分别为2.1×10-22和6.3×10-18。因此,当足量的Na2S加入含有As(Ⅲ)、Fe2+的溶液中时,即可迅速发生反应生成沉淀,而且金属离子的脱除率可高达99%以上,主要的反应如下:
Na2S+H2SO4=H2S+Na2SO4 (1)
3H2S+2H3AsO3=As2S3↓+6H2O (2)
H2S+Fe2+=FeS↓+2H+ (3)
经实验研究,硫化沉砷的最佳条件如下:终点pH=4.0,n(S2-):n(As)=3.0:1,反应温度为25 ℃,反应时间为60 min。在此条件下,硫化沉砷的沉淀率可高达99.65%,硫化沉淀含砷59.19%,含硫36.87%。对该沉淀进行SEM-EDS、XRD表征,结果见图2。
表2 含砷废水的化学成分
Table 2 Composition of arsenic containing wastewater (mg/L)

由图2(a)可知,硫化沉淀渣的X射线衍射峰弱且宽,说明该沉淀是无定形的。SEM结果见图2(b)和(c),可知无定形As2S3沉淀渣主要由有众多松散、细小的絮状颗粒部分附着在大颗粒表面组成,也有部分小颗粒相互粘附,整体呈现松散、多孔状。EDS结果见图2(d),可知该沉淀含砷63.0%,含硫37.0%,n(S):n(As)= 1:1.4,接近As2S3中硫、砷原子比,因此可确定硫化沉淀为无定形As2S3。按照TCLP流程对硫化砷沉淀做了毒性检测,砷的浸出浓度达到212.9 mg/L,属于国标GB 5085.7-2007中危险固废名录HW24中的危险固废,需要进一步处理。
2.3 As-S系一元水热还原矿化稳定砷
为了改善三硫化二砷沉淀的稳定性,采用水热还原矿化工艺模拟雌黄/雄黄的低温热液的成矿环境,期望能使三硫化二砷沉淀转化为雌黄或雄黄矿物。

图2 硫化沉淀渣的表征结果
Fig. 2 Characterization results of As2S3
在水热还原矿化过程中,称取一定质量的As2S3沉淀,再称取As2S3沉淀质量的5%的葡萄糖至水热反应釜内,用浓度为6%的Na2SO4溶液调浆,使水热反应釜的填充度达到70%,密封后放入烘箱升温到240 ℃后保温12 h。反应结束后冷却至室温后,对矿化后的As2S3渣烘干后进行检测。
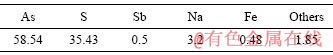
对水热渣进行XRD、SEM-EDS表征,结果见图3。由图3(a)可知,经水热还原矿化的As2S3沉淀渣的晶型出现了明显的变化,水热渣的衍射峰与As2S3晶体(PDF:71-2435)的特征峰完全对应,表明无定形的As2S3经水热还原后转化为的As2S3晶体,即雌黄。SEM-EDS检测结果见图3(b)和(c),在低倍SEM图中,几乎未见细小、松散的小颗粒,且有大量粒径大于100 μm的雌黄晶体块;在高倍SEM图中,晶体块表面致密、较光滑,有少许凹陷的坑。EDS结果显示沉淀含砷61.4%,含硫38.7%。雌黄晶体的TCLP毒性检测浸出浓度为3.86 mg/L,与As2S3沉淀的浸出毒性结果相比显著降低,说明水热矿化后的As2S3晶体稳定性显著提升。经过水热转化后的As2S3晶体含砷58.54%,含硫35.43%(见表3)。
由于雌黄的含砷量高于其他稳定化矿物,因此雌黄的体量远远小于其他稳定化矿物,这将大大节省填埋成本。
表3 一元水热还原矿化渣的化学成分
Table 3 Chemical composition of slag from unary hydrothermal reduction and mineralization (mass fraction, %)
2.4 As-Fe-S系二元水热还原矿化稳定砷
根据2.3试验结果可见,无定形的As2S3沉淀经过水热还原矿化后,转化为了雌黄晶体,且稳定性显著改善。由于砷与铁、硫常以毒砂矿物的形式存在于自然界,即使雌黄、雄黄等含砷矿物,也常与FeS、黄铁矿等伴生,且也有利用铁盐沉淀、吸附脱砷的研究。因此,借鉴砷地球化学成矿规律,提出As-Fe-S系二元水热矿化稳定砷的工艺,期望能以砷黄铁矿形式实现砷的稳定化。
在水热还原矿化过程中,先称取一定质量的As2S3沉淀,再按n(Fe):n(As)=1:3加入FeS沉淀(现用现制),再称取As2S3沉淀质量的5.0%的葡萄糖至水热反应釜内,用质量分数为6%的Na2SO4溶液调浆,使水热反应釜的填充度达到70%,密封后放入烘箱升温到240 ℃后保温12 h。反应结束自然后冷却至室温后,对水热渣烘干后进行检测。
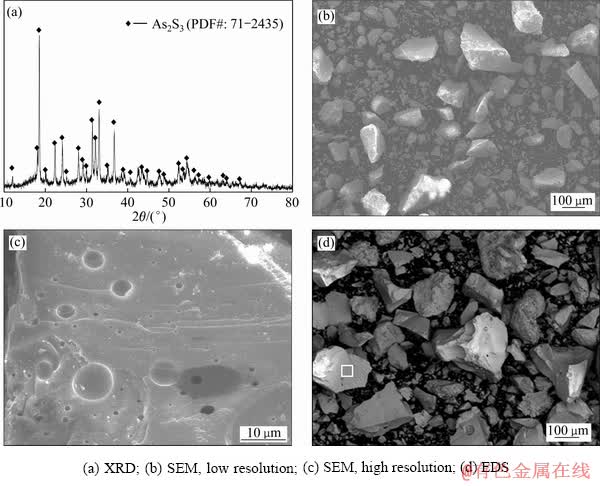
图3 一元水热还原矿化渣的表征结果
Fig. 3 Characterization results of slag from unary hydrothermal reduction and mineralization

图4 二元水热还原矿化渣的表征结果
Fig. 4 Characterization results of slag from binary hydrothermal reduction and mineralization
对二元水热矿化渣进行XRD与SEM-EDS表征,结果见图4。由图4(a)可知,无定形As2S3沉淀与FeS沉淀混合后进行水热矿化,得到的仍然是As2S3晶体,其X射线衍射峰与As2S3晶体(PDF:71-2435)的特征峰完全对应,但是没有检测其他晶体的衍射峰,说明在此条件下得到是雌黄-铁硫系(黄铁矿、硫化亚铁)混合物。SEM检测结果见图4(b)和(c),在低倍SEM图中,松散的小颗粒相互团聚在一起,且有大量粒径50 μm左右的团聚块;在高倍SEM图中,颗粒块表面较致密,有少量细微横纹。EDS结果显示,团聚块表面较暗的部分(A)砷、铁、硫的含量依次为21.1%、47.7%和31.2%,较亮的部分(B)砷、硫的含量为57.3%、38.2%,而铁含量仅为4.6%。XRF检测结果见表4,二元水热还原矿化渣含砷45.37%。对二元水热渣的浸出毒性进行了检测,砷的浸出浓度为2.65 mg/L。与一元水热还原矿化渣相比,砷的浸出毒性进一步降低,也达到了提升As2S3沉淀稳定性的目的,为含砷、铁的废水中砷的脱除及稳定化提供了新的研究思路。
表4 二元水热还原矿化渣的化学成分
Table 4 Chemical composition of slag from binary hydrothermal reduction and mineralization (mass fraction, %)

3 结论
1) 硫化沉淀法脱砷的沉淀率可达到99.65%,得到的无定形As2S3沉淀;在TCLP毒性检测中的砷浓度高达212.9mg/L,经过水热还原矿化后,无定形的As2S3沉淀转化为As2S3晶体,其砷含量可达58.5%,砷的浸出浓度可降至3.86 mg/L,低于危险废弃物中砷的浸出浓度限值5.0 mg/L。
2) 采用As-Fe-S系二元水热矿化工艺处理铜冶炼污酸,砷以雌黄-铁硫系(黄铁矿、硫化亚铁)混合物的形式实现了稳定化。稳定化产物在TCLP毒性检测中砷的浓度为2.65 mg/L。
3) 采用硫化沉砷-As-S系一元水热还原矿化及As-Fe-S系二元水热矿化工艺处理高砷废水的优点在于:①采用硫化沉淀法高效脱除高砷废水中的砷;②采用水热还原矿化工艺将无定形的As2S3沉淀转化为雌黄矿物,提高了As2S3沉淀的稳定性,显著降低了砷溶解毒性;③雌黄矿物稳定性好,含砷量高,有利于大幅降低稳定化产物的增容比,减少填埋空间需求。
REFERENCES
[1] DRAHOTA P, FILIPPI M. Secondary arsenic minerals in the environment: A review[J]. Environment International, 2009, 35 (8): 1243-1255.
[2] LI Yuan-cheng, MIN Xiao-bo, CHAI Li-yuan, SHI Mei-qing, TANG Chong-jian, WANG Qing-wei, LIANG Yan-jie, LEI Jie, LIYANG Wen-Jun. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals[J]. Journal of Environmental Management, 2016, 181: 756-761.
[3] 徐宝强, 史腾腾, 杨 斌, 杨 佳, 蒋文龙. 含砷烟尘的处理及利用研究现状[J]. 昆明理工大学学报(自然科学版), 2019, 44(1): 1-11.
XU Bao-qiang, SHI Teng-teng, YANG Bin, YANG Jia, JIANG Wen-long. Research status on treatment and utilization of arsenic containing dust[J]. Journal of Kunming University of Science and Technology (Natural Science), 2019, 44(1): 1-11.
[4] 郑雅杰, 张胜华, 龚 昶. 含砷污酸资源化回收铜和砷的新工艺[J]. 中国有色金属学报, 2013, 23(10): 2985-2992.
ZHENG Ya-jie, ZHANG Sheng-hua, GONG Chang. Novel technique for recovery of copper and arsenic from arsenic- containing waste acid[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(10): 2985-2992.
[5] 郑雅杰, 刘万宇, 白 猛, 张传福. 采用硫化砷渣制备三氧化二砷工艺[J]. 中南大学学报(自然科学版), 2008, 39(6): 1157-1163.
ZHENG Ya-jie, LIU Wan-yu, BAI Meng, ZHANG Chuan-fu. Preparation of arsenic trioxide from arsenic sulfide slag[J]. Journal of Central South University (Science and Technology), 2008, 39(6): 1157-1163.
[6] 侯汉娜, 陈甜甜. 硫化砷渣全湿法制备单质砷的研究[J]. 环境保护科学, 2014, 40(6): 42-45.
HOU Han-na, CHEN Tian-tian. Experimental study of elemental arsenic production by the wet process of arsenic sulfide residue[J]. Environmental Protection Science, 2014, 40(6): 42-45.
[7] YU Guo-lin, ZHANG Ying, ZHENG Shi-li, ZOU Xing, WANG Xiao-hui, ZHANG Yi. Extraction of arsenic from arsenic-containing cobalt and nickel slag and preparation of arsenic-bearing compounds[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1918-1927.
[8] 宣之强. 中国砷矿资源概述[J]. 化工矿产地质, 1998, 20(3): 8-14.
XUAN Zhi-qiang. A brief account of Chinese arsenic resources[J]. Geology of Chemical Minerals. 1998, 20(3): 8-14.
[9] 关大明. 铜冶炼污酸浓缩液资源化处理实验研究[D]. 赣州: 江西理工大学, 2015.
GUAN Da-ming. Recycling of acidic wastewater from copper smelting[D]. Ganzhou: Jiangxi University of Science and Technology, 2015.
[10] 汪海涛. 含砷废渣的稳定化/固化处理研究[D]. 武汉: 武汉理工大学, 2007.
WANG Hai-tao. Study on the stabilization/ solidification of arsenic slag[D]. Wuhan: Wuhan University of Technology, 2007.
[11] 聂永丰. 固体废物处理工程技术手册[M]. 北京: 化学工业出版社, 2012.
NIE Yong-feng. Handbook on solid waste management and technology[M]. Beijing: Chemical Industry Press, 2012.
[12] LI Yu-hu, LIU Zhi-hong, LI Qi-hou, ZHAO Zhong-wei, LIU Zhi-yong, ZENG Li, LI Li. Removal of arsenic from arsenate complex contained in secondary zinc oxide[J]. Hydrometallurgy, 2011, 109(3/4): 237-244.
[13] 陶青英. 含砷废水的石灰沉淀法处理及溶液化学研究[D]. 武汉: 武汉科技大学, 2011.
TAO Qing-ying. Study on the technic of arsenic removal by lime precipitation and the mechanism of the solution chemistry[D]. Wuhan: Wuhan University of Science and Technology, 2011.
[14] NAZARI A M, RADZINSKI R, GHAHREMAN A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic[J]. Hydrometallurgy, 2017, 174: 258-281.
[15] 李玉虎. 有色冶金含砷烟尘中砷的脱除与固化[D]. 长沙: 中南大学, 2012.
LI Yu-hu. Arsenic removal and solidification of arsenic bearing dusts of nonferrous metallurgy[D]. Changsha: Central South University, 2012.
[16] PALFY P, VIRCIKOVA E, MOLNAR L. Processing of arsenic waste by precipitation and solidification[J]. Waste Management, 1999, 19 (1): 55-59.
[17] 张 洁, 王兴润, 张增强, 农泽喜, 刘 锋, 何 洁, 于泓锦. 不同添加组分对高温烧结含砷废渣中砷环境释放行为的影响[J]. 西北农林科技大学学报(自然科学版), 2013, 41(6): 91-97.
ZHANG Jie, WANG Xing-run, ZHANG Zeng-qiang, NONG Ze-xi, LIU Feng, HE Jie, YU Hong-jin. Effect of different additives on environmental release behavior of arsenic during sintering of arsenic-containing waste[J]. Journal of Northwest A&F University (Nat. Sci. Ed.), 2013, 41(6): 91-97.
[18] 向雪松, 柴立元, 闵小波, 张 盈, 邓 荣, 姜文英. 砷碱渣浸出液铁盐沉砷过程研究[J]. 中国锰业, 2006, 24(1): 30-33.
XIANG Xue-song, CHAI Li-yuan, MIN Xiao-bo, ZHANG Ying, DENG Rong, JIANG Wen-ying. A study on the removal of arsenic from leaching liquor of arsenic- containing alkaline dregs by precipitation as ferric arsenates[J]. China’s Manganese Industry, 2006, 24(1): 30-33.
[19] SONG S, LOPEZ-VALDIVIESO A, HERNANDEZ- CAMPOS D J, PENG C, MONROY-FERNANDEZ M G, RAZO-SOTO I. Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite[J]. Water Research, 2006, 40(2): 364-372.
[20] 朱义年, 张学洪, 解庆林, 陈余道, 王敦球, BRODER M. 砷酸盐的溶解度及其稳定性随pH值的变化 [J]. 环境化学, 2003, 22(5): 478-484.
ZHU Yi-nian, ZHANG Xue-hong, XIE Qing-lin, CHEN Yu-dao, WANG Dun-qiu, BRODER M. Dependence of arsenate solubility and stability on pH value[J]. Environmental Chemistry, 2003, 22(5): 478-484.
[21] LEWIS A E. Review of metal sulphide precipitation[J]. Hydrometallurgy, 2010, 104(2): 222-234.
[22] HARRIS B. The removal of arsenic from process solutions: Theory and industrial practice[M]. New York: John Wiley & Sons, Inc., 2003: 1888-1902.
[23] LE PAPE P, BATTAGLIA-BRUNET F, PARMENTIER M, JOULIAN C, GASSAUD C, FERNANDEZ-ROJO L, GUIGNER J M, IKOGOU M, STETTEN L, OLIVI L, CASIOT C, MORIN G. Complete removal of arsenic and zinc from a heavily contaminated acid mine drainage via an indigenous SRB consortium[J]. Journal of Hazardous Materials, 2017, 321: 764-772.
[24] HUISMAN J L, SCHOUTEN G, SCHULTZ C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry[J]. Hydrometallurgy, 2006, 83(1/4): 106-113.
[25] CRUZ V C, PAGNANELLI F, CIBATI A, UCCELLETTI D, PALLESCHI C, TORO L. Biotreatment and bioassessment of heavy metal removal by sulphate reducing bacteria in fixed bed reactors[J]. Water Research, 2010, 44(1): 151-158.
[26] 成应向, 宋伟龙, 许友泽, 戴友芝, 邱亚群, 王强强. Na2S与高聚复配絮凝剂处理酸性高As废水[J]. 环境科学研究, 2013, 26(9): 1007-1013.
CHENG Ying-xiang, SONG Wei-long, XU You-ze, DAI You-zhi, QIU Ya-qun, WANG Qiang-qiang. Study on treatment of acidic high-arsenic wastewater with Na2S and polymer composite flocculants[J]. Research of Environmental Sciences, 2013, 26(9): 1007-1013.
[27] JIA Yong-feng, ZHANG Dan-ni, PAN Rong-rong, XU Li-ying, DEMOPOULOS George-P. A novel two-step coprecipitation process using Fe(Ⅲ) and Al(Ⅲ) for the removal and immobilization of arsenate from acidic aqueous solution[J]. Water Research, 2012, 46(2): 500-508.
[28] NISHIMURA T, ROBINS R G. A re-evaluation of the solubility and stability regions of calcium arsenites and calcium arsenates in aqueous solution at 25 ℃[J]. Mineral Processing & Extractive Metallurgy Review, 1998, 18(3/4): 283-308.
[29] DU Ying, LU Qiong, CHEN Hui-yun, DU Ya-guang, DU Dong-yun. A novel strategy for arsenic removal from dirty acid wastewater via CaCO3-Ca(OH)2-Fe(Ⅲ) processing[J]. Journal of Water Process Engineering, 2016, 12: 41-46.
[30] ZHANG Gao-sheng, QU Jiu-hui, LIU Hui-juan, LIU Rui-ping, WU Rong-cheng. Preparation and evaluation of a novel Fe-Mn binary oxide adsorbent for effective arsenite removal[J]. Water Research, 2007, 41(9): 1921-1928.
[31] LIU Rui-ping, SUN Li-hua, QU Jiu-hui, LI Gui-bai. Arsenic removal through adsorption, sand filtration and ultrafiltration: In situ precipitated ferric and manganese binary oxides as adsorbents[J]. Desalination, 2009, 249(3): 1233-1237.
[32] CHOONG T S Y, CHUAH T G, ROBIAH Y, GREGORY KOAY F L, AZNI I. Arsenic toxicity, health hazards and removal techniques from water: An overview[J]. Desalination, 2007, 217(1/3): 139-166.
[33] 陆俏利, 瞿广飞, 吴 斌, 宁 平. 矿区含砷尾矿及废渣稳定化研究[J]. 环境工程学报, 2016, 10(5): 2587-2594.
LU Qiao-li, QU Guang-fei, WU Bin, NING Ping. Study on stabilization of arsenic tailings and waste residue[J]. Chinese Journal of Environmental Engineering, 2016, 10(5): 2587-2594.
[34] LIANG Yan-jie, MIN Xiao-bo, CHAI Li-yuan, WANG Mi, LIYANG Wen-jun, PAN Qing-lin, OKIDO M. Stabilization of arsenic sludge with mechanochemically modified zero valent iron[J]. Chemosphere, 2017, 168: 1142-1151.
[35] 徐 慧, 闵小波, 梁彦杰, 王云燕. 机械力活化Fe-MnO2稳定含砷废渣[J]. 中国有色金属学报, 2017, 27(10): 2170-2179.
XU Hui, MIN Xiao-bo, LIANG Yan-jie, WANG Yun-yan. Stabilization of arsenic bearing solid waste with Fe-MnO2 activated by mechanochemical process[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(10): 2170-2179.
[36] MAJZLAN J, DRAHOTA P, FILIPPI M, GREVEL K D, KAHL W A, PLA IL J, BOERIO-GOATES J, WOODFIELD B F. Thermodynamic properties of scorodite and parascorodite (FeAsO4·2H2O), kaňkite (FeAsO4·3.5H2O), and FeAsO4[J]. Hydrometallurgy, 2012, 117/118: 47-56.
[37] LANGMUIR D, MAHONEY J, ROWSON J. Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4·2H2O) and their application to arsenic behavior in buried mine tailings[J]. Geochimica et Cosmochimica Acta, 2006, 70(12): 2942-2956.
[38] FUJITA T, TAGUCHI R, ABUMIYA M, MATSUMOTO M, SHIBATA E, NAKAMURA T. Effect of pH on atmospheric scorodite synthesis by oxidation of ferrous ions: Physical properties and stability of the scorodite[J]. Hydrometallurgy, 2009, 96(3): 189-198.
[39] MIN Xiao-bo, LIAO Ying-ping, CHAI Li-yuan, YANG Zhi-hui, XIONG Shan, LIU Lin, LI Qing-zhu. Removal and stabilization of arsenic from anode slime by forming crystal scorodite[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1298-1306.
[40] MONHEMIUS A J, SWASH P M. Removing and stabilizing As from copper refining circuits by hydrothermal processing[J]. JOM, 1999, 51(9): 30-33.
[41] 余自秀, 李存兄, 魏 昶, 樊 刚, 李兴彬, 邓志敢, 李旻廷. 砷铁水热共沉淀制备大颗粒臭葱石 [J]. 过程工程学报, 2018, 18(1): 126-132.
YU Zi-xiu, LI Cun-xiong, WEI Chang, FAN Gang, LI Xing-bin, DENG Zhi-gan, LI Min-ting. Preparation of large-sized scorodite based on arsenic and iron hydrothermal co-precipitation[J]. The Chinese Journal of Process Engineering, 2018, 18(1): 126-132.
[42] KITAMURA Y, OKAWA H, KATO T, SUGAWARA K. Effect of reaction temperature on the size and morphology of scorodite synthesized using ultrasound irradiation[J]. Ultrasonics Sonochemistry, 2017, 35: 598-604.
[43] FUJITA T, TAGUCHI R, ABUMIYA M, MATSUMOTO M, SHIBATA E, NAKAMURA T. Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part II. Effect of temperature and air[J]. Hydrometallurgy, 2008, 90(2/4): 85-91.
[44] FUJITA T, TAGUCHI R, ABUMIYA M, MATSUMOTO M, SHIBATA E, NAKAMURA T. Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. PartⅠ[J]. Hydrometallurgy, 2008, 90(2/4): 92-102.
[45] 刘志宏, 杨校锋, 刘智勇, 李玉虎, 李启厚. 制备方法对臭葱石浸出稳定性的影响[J]. 过程工程学报, 2015, 15(3): 412-417.
LIU Zhi-hong, YANG Xiao-feng, LIU Zhi-yong, LI Yu-hu, LI Qi-hou. Effects of synthesis methods for scorodite on its leaching stability[J]. The Chinese Journal of Process Engineering, 2015, 15(3): 412-417.
[46] BLUTEAU M C, BECZE L, DEMOPOULOS G P. The dissolution of scorodite in gypsum-saturated waters: Evidence of Ca-Fe-AsO4 mineral formation and its impact on arsenic retention[J]. Hydrometallurgy, 2009, 97(3/4): 221-227.
[47] BLUTEAU M C, DEMOPOULOS G P. The incongruent dissolution of scorodite—Solubility, kinetics and mechanism[J]. Hydrometallurgy, 2007, 87(3/4): 163-177.
[48] 刘 璟, 黄 晰, 谌 书, 刘 娟, 武长虹. 人工合成图水羟砷铁矾的矿物学研究[J]. 岩石矿物学杂志, 2012, 31(6): 901-906.
LIU Jing, HUANG Xi, CHEN Shu, LIU Juan, WU Chang-hong. Mineralogical research on synthesized tooeleite[J]. Acta Petrologica et Mineralogica, 2012, 31(6): 901-906.
[49] LIU Jing, CHENG Hong-fei, FROST R L, DONG Fa-qing. The mineral tooeleite Fe6(AsO3)4SO4(OH)4·4H2O—An infrared and Raman spectroscopic study-environmental implications for arsenic remediation[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2013, 103: 272-275.
[50] MAJZLAN J, DACHS E, BENISEK A, KOCH C B, BOLANZ R, GOTTLICHER J, STEININGER R. Thermodynamic properties of tooeleite, Fe63+(As3+O3)4(SO4)- (OH)4·4H2O[J]. Chemie der Erde-Geochemistry, 2016, 76(3): 419-428.
[51] CHAI Li-yuan, YUE Meng-qing, YANG Jin-qin, WANG Qing-wei, LI Qing-zhu, LIU Hui. Formation of tooeleite and the role of direct removal of As(Ⅲ) from high-arsenic acid wastewater[J]. Journal of Hazardous Materials, 2016, 320: 620-627.
[52] 朱 琳. 砷羟基磷灰石的制备及其砷毒性浸出研究[D]. 昆明: 昆明理工大学, 2015.
ZHU Lin. Study on the preparation and leachability of arsenic hydroxyapatite[D]. Kunming: Kunming University of Science and Technology, 2015.
[53] VINALS J, SUNYER A, MOLERA P, CRUELLS M, LLORCA N. Arsenic stabilization of calcium arsenate waste by hydrothermal precipitation of arsenical natroalunite[J]. Hydrometallurgy, 2010, 104(2): 247-259.
[54] SUNYER A, VINALS J. Arsenate substitution in natroalunite: A potential medium for arsenic immobilization. Part 1: Synthesis and compositions[J]. Hydrometallurgy, 2011, 109(1/2): 54-64.
[55] SUNYER A, VINALS J. Arsenate substitution in natroalunite: A potential medium for arsenic immobilization. Part 2: Cell parameters and stability tests[J]. Hydrometallurgy, 2011, 109(1/2): 106-115.
[56] 陶志超. 水热合成砷方钠石及其毒性浸出特性的研究[D]. 昆明: 昆明理工大学, 2016.
TAO Zhi-chao. Study on the hydrothermal preparation and leachability of arsenic sodalite[D]. Kunming: Kunming University of Science and Technology, 2016.
[57] 赵珊茸. 结晶学及矿物学[M]. 北京: 地址出版社. 2002.
ZHAO Shan-rong. Crystallography and mineralogy[M]. Beijing: Geologica Publishing House, 2002.
[58] YANG Tian-zu, HU Bin, LIU Wei-feng, ZHANG Du-chao, CHEN Lin. A novel process for the treatment of copper- smelting waste acid with a high arsenic concentration[J]. JOM, 2018, 70(10): 2022-2026.
[59] 刘守庆, 罗中秋, 和 森, 周新涛, 贾庆明. 高炉矿渣-粉煤灰地聚合物胶凝材料固化砷钙渣[J]. 化工进展, 2017, 36(7): 2660-2666.
LIU Shou-qing, LUO Zhong-qiu, HE Sen, ZHOU Xin-tao, JIA Qing-min. Solidification/ stabilization of calcium arsenate waste with blast furnace slag and fly ash geopolymer materials[J]. Chemical Industry And Engineering Progress, 2017, 36(7): 2660-2666.
[60] SWASH P M, MONHEMIUS A J. Comparison of the solubilities of arsenic-bearing wastes from hydro- metallurgical and pyrometallurgical processes[J]. GDMB, 2000, 83: 141-152.
[61] 罗中秋. 钠明矾石沉淀除砷应用基础研究[D]. 昆明: 昆明理工大学, 2015.
LUO Zhong-qiu. Application research on arsenic immobilization through natroalunite phase[D]. Kunming: Kunming University of Science and Technology, 2015.
HU Bin, YANG Tian-zu, LIU Wei-feng, ZHANG Du-chao, CHEN Lin
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: Arsenic is one of paragenetic and associated elements in non-ferrous minerals, which is enriched in “the three wastes” containing arsenic during the smelting of nonferrous metal processes. The safety disposal of wastewater containing arsenic has troubled nonferrous metals smelting enterprises due to the strong carcinogenicity and toxicity of arsenic. This paper firstly introduced the sources and harm of wastewater arsenic-containing. And then the stabilization technologies including chemical stabilization and mineral stabilization were reviewed. By comparing the advantages and disadvantages of stabilization technologies for treating wastewater containing arsenic, and referring to the mineralization rule of arsenic in geochemistry, a sulfidization-hydrothermal reduction mineralization process of arsenic was proposed. The results show that, firstly, 99.65% arsenic in wastewater containing arsenic was precipitated in the form of amorphous As2S3 by using Na2S. Then the leachate concentration of arsenic of amorphous As2S3 in TCLP test is 212.97 mg/L. Next, the hydrothermal reduction mineralization process in As-S unary system and hydrothermal reduction mineralization process in As-Fe-S binary system are adopted to transform amorphous As2S3 to orpiment and orpiment and iron-sulfur system (pyrite, ferrous sulfide) mixture separately. And the As leachate concentration of these corresponding hydrothermal slags in the TCLP test is reduced to 3.86 mg/L, 2.65 mg/L separately. A satisfied result of removal and stabilization of arsenic from wastewater are achieved by the novel processes, which provides a promising way to remove and stabilize arsenic from high arsenic containing wastewater.
Key words: arsenic; arsenic trisulfide; stabilization; hydrothermal; mineralization
Foundation item: Projects(2018YFC1901605, 2018YFC1901604) supported by the National Key Research and Development Program of China; Project(201806375047) supported by the Visiting Scholar of China Scholarship Council; Project(51404296) supported by the Young Scientists Fund of the National Natural Science Foundation of China
Received date: 2019-04-06; Accepted date: 2019-12-13
Corresponding author: LIU Wei-feng; Tel: +86-13548654403; E-mail: liuweifeng@csu.edu.cn
(编辑 何学锋)
基金项目:国家重点研发计划资助项目(2018YFC1901605,2018YFC1901604);国家留学基金委访问学者项目(201806375047);国家自然科学青年基金资助项目(51404296)
收稿日期:2019-04-06;修订日期:2019-12-13
通信作者:刘伟锋,副教授,博士;电话:13548654403;E-mail:liuweifeng@csu.edu.cn

