文章编号:1004-0609(2016)-04-0878-06
油酸钠作用下异极矿的浮选行为及作用机理
刘 诚,冯其明,张国范
(中南大学 资源与生物工程学院,长沙 410083)
摘 要:通过纯矿物试验研究油酸钠为捕收剂体系中异极矿的浮选行为。结果表明:当油酸钠用量为3×10-4 mol/L、pH值为4~8和11时,异极矿浮选回收率均在80%左右。Zeta电位及红外光谱测试结果表明:油酸钠在异极矿表面主要发生化学吸附,同时也可能存在物理吸附。根据油酸钠溶液和锌离子水解组分含量化学计算,当pH值为4~8时,油酸钠溶液的优势组分为RCOO-和(RCOO)22-;而异极矿表面锌离子主要以Zn2+和少量ZnOH+形式存在。结合异极矿在油酸钠捕收剂体系中的浮选行为,油酸钠在异极矿表面的相互作用原理是异极矿表面Zn2+和羟基络合物Zn(OH)+成为浮选的活性质点,能与油酸钠作用形成油酸盐,从而使异极矿疏水上浮;RCOOH(aq)分子和离子-分子缔合物RCOOH·RCOO-的物理吸附也可能存在;而在pH为11时矿物可浮性较好,可能是油酸根离子与矿物表面的形成Zn(OH)2发生离子交换。
关键词:异极矿;油酸钠;浮选
中图分类号:TD91 文献标志码:A
锌做为一种重要有色金属广泛应用于镀锌、锌电池和汽车等行业[1-2],消费量仅次于铁铝铜消费量。锌矿常和铅矿伴生共存,而获得锌精矿产品最传统的工艺是通过浮选方式[3]。近年随着硫化锌矿物的日益枯竭,异极矿的回收日益受到重视,目前异极矿的提取主要是采取湿法冶金的方法[4-5]。异极矿属于硅酸盐矿物,极易泥化,分选困难。因此,很少有关异极矿浮选的文献报导[6]。
在浮选过程中,捕收剂在固-液界面的吸附与浮选的关系一直受到矿物加工研究者的关注,油酸钠属于脂肪酸盐,常作为阴离子捕收剂被广泛应用于氧化矿和硅酸盐矿物的浮选中[7],如钛铁矿[8]、萤石[9]、赤铁矿[10]、一水硬铝石等[11]。有关油酸对矿物的作用机理国内外有很多文献报道,主要可以归为以下几种情况:油酸根离子与矿物晶格中金属离子或者矿物表面的金属离子羟基络合物发生化学作用形成金属油酸 盐[12];油酸根离子与矿物表面发生离子交换[13];油酸分子、离子缔合物在矿物表面发生物理吸附[14];化学吸附和物理吸附共存[15]。
异极矿属于硅酸盐矿物,表面具有强亲水性,疏基捕收剂浮选效果很差,即便用硫酸铜活化后浮选回收率也较低[16],而关于油酸钠浮选异极矿的机制的文献鲜见报道。本文作者通过研究油酸钠对异极矿的捕收性能,并综合考虑矿物表面化学和捕收剂溶液化学,运用动电位测试和红外光谱测试等分析手段,系统讨论油酸钠对异极矿的捕收机理,为异极矿的浮选提供理论依据。
1 实验
1.1 实验原料
本试验中所用异极矿取自湖南某宝石市场,经手选、破碎、干式瓷球磨后筛分后,得到粒径小于0.074 mm粒级的纯矿物,对样品进行XRD谱分析(见图1),由图1可知,未检测到杂质峰,说明异极矿的纯度极高,达到98%以上,符合纯矿物实验的要求。矿浆pH调整剂为硫酸和氢氧化钠,均为分析纯,油酸钠为化学纯。实验用水为一次蒸馏水。
1.2 浮选实验
浮选实验采用容积为40 mL的XFG 型挂槽式浮选机,浮选温度为25 ℃。每次实验称取矿样2 g 置于浮选槽内,加入一定量蒸馏水搅拌1 min 后加 pH调整剂搅拌2 min,加入捕收剂搅拌3 min 后测定 pH值,浮选 3 min。浮选过程采取手工刮泡,浮选完成后将刮出所得的精矿(泡沫产品)和尾矿(槽内产品)经烘干、称量、计算产率。纯矿物试验所刮出的泡沫产品产率等于浮选回收率。

图1 实验样品XRD谱
Fig. 1 XRD pattern of flotation sample
1.3 红外测试
红外光谱测试在 Nicolet NEXUS 670型傅里叶变换红外光谱仪上进行测定。采用 KBr 压片,测量范围为4500~400 cm-1。红外分析样品制备过程如下:每次称取适量矿样放入浮选槽,用 NaOH 或 H2SO4调节矿浆pH 后加入一定量油酸钠溶液,充分搅拌后进行固液分离,用相同 pH 的蒸馏水溶液洗涤矿物2 次,所得样品在 60℃以下烘干,进行红外光谱检测。
1.4 Zeta电位测试
采用Coulter Delsa440sx Zeta型电位分析仪进行Zeta电位测试。用高精度天平称取30 mg粒度磨制小于2 μm的单矿物样品,放入烧杯中并加入50 mL蒸馏水,调节溶液pH值并添加相关浮选药剂后搅拌3 min,然后放入样品池中进行Zeta电位测定,每个实验条件测量3次后取平均值。实验所用电解质为0.001 mol/L的KNO3溶液。
2 结果与讨论
2.1 实验结果
图2所示为不同pH值条件下油酸钠对异极矿的可浮性影响。从图2结果可知,在pH值为5~8时,异极矿的可浮性较好,在其他pH值范围,回收率均有不同程度的下降,pH值从8升到10时回收率下降30%左右,在pH值为11时也可以获得较好的回收率,pH值为11~12时矿物回收率迅速下降;图3所示为当 pH 值为7~7.5时油酸钠用量与矿物回收率的关系。从图3结果可知,当油酸钠用量为 0~1×10-4 mol/L时,异极矿的回收率快速提高;当油酸钠用量为1×10-4~ 2×10-4 mol/L时,回收率增加缓慢。进一步增加其用量,回收率再次呈现出快速增长趋势;当油酸钠用量大于3×10-4 mol/L时,回收率增加缓慢。由此可见,在适当的条件下用油酸钠浮选异极矿时能取得较好浮选回收率。

图2 油酸钠浓度3×10-4 mol/L时pH值对异极矿回收率的影响
Fig. 2 Flotation recovery of hemimorphite as function of pH with 3×10-4 mol/L sodium oleate
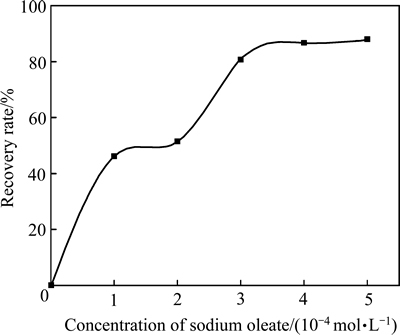
图3 pH为7.0~7.5时油酸钠用量对异极矿可浮性影响
Fig. 3 Flotation recovery of hemimorphite with dosage of sodium oleate at pH of 7.0-7.5
2.2 捕收机理
2.2.1 异极矿的表面电性
图4所示为矿物的Zeta电位与pH 值的关系。从图 4 可知,以盐酸和氢氧化钠调节 pH 值时,异极矿零电点在pH 值为5.1 左右,和Rank Brothers测试的结果5.4相近,但与Mular和Roberts测试的结果6.9相差甚远[17-18]。加入油酸钠后在相同 pH 值下矿物的Zeta 电位发生负移;零电点由 5.1 降到 2.3 左右,当pH<2.3 时,电位为正且相对于原来发生负移,说明油酸钠在异极矿表面发生了吸附,可能为静电吸附,也可能为化学吸附;当 pH>2.3 时,加入油酸钠使相同 pH 值下矿物的 Zeta电位发生负移,特别是在异极矿可浮性较好的 pH 区间内,电位降低的幅度更大,说明油酸根在异极矿表面发生吸附。与矿物表面带同种电荷的油酸根能很好地吸附在矿物表面,可以认为是油酸离子吸附到矿物亥姆霍兹层表面[19],此时油酸根与异极矿之间的作用力不以静电力为主,可能发生了化学作用。

图4 异极矿Zeta电位与pH值变化关系
Fig. 4 Zeta potential of hemimorphite as a function of pH
2.2.2 矿物红外光谱分析
图5所示为异极矿与油酸钠作用前后的红外光谱测试结果。在400~4000 cm-1范围内异极矿出现了数个明显特征峰,主要是—OH和Si—O的吸收峰[20]。而油酸钠的红外光谱图中,2921.3 cm-1和2851.2 cm-1分别为油酸钠中—CH2—和—CH3中C—H键的对称振动吸收峰,1716.2 cm-1、1559.2 cm-1、1446.5 cm-1和1424.9 cm-1是R—COOH中—COO—基团的特征吸收峰,721.8 cm-1吸收峰是其面内弯曲振动吸收峰[21-24]。由图5可知,异极矿经油酸钠处理后红外光谱发生了显著变化,除了异极矿自身的特征吸收峰外,还在 2923.1 cm-1和2851.2 cm-1处出现了新的吸收峰,与油酸钠中—CH2—和—CH3中C—H键的对称振动吸收峰相对应,说明油酸钠吸附于异极矿表面,同时在1594.6 cm-1和1456.4 cm-1处出现了新的吸收峰,与油酸钠光谱中的1446.5 cm-1羧基的特征峰相比,波数向高波段分别移动约35和10 cm-1,说明油酸钠与异极矿发生了化学吸附。
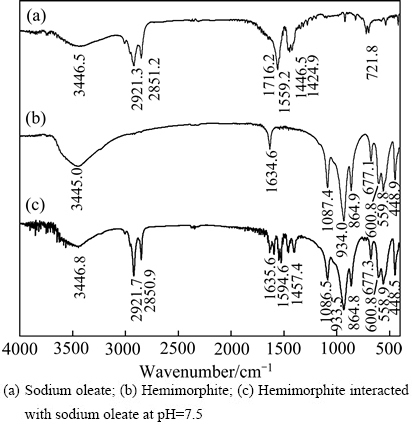
图5 油酸钠与异极矿作用前后的红外光谱
Fig. 5 Infrared spectra of sodium oleate and hemimorphite
2.2.3 异极矿表面的锌离子在溶液中的形态
由金属离子水解平衡原理可知,异极矿矿物表面溶出的锌离子在水溶液中会发生水解平衡,生成各种锌的羟基络合物,优势组分和pH值紧密相关,锌离子在水溶液中主要有以下羟基络合反应[25-26]:
Zn(OH)2(s) Zn2++2OH-, Ksp=1×10-15.76 (1)
Zn2++2OH-, Ksp=1×10-15.76 (1)
Zn2++OH-  Zn(OH)+, β1=1×105.0 (2)
Zn(OH)+, β1=1×105.0 (2)
Zn2++2OH-  Zn(OH)2(aq), β2=1×1011.1 (3)
Zn(OH)2(aq), β2=1×1011.1 (3)
Zn2++3OH- 
 , β3=1×1013.6 (4)
, β3=1×1013.6 (4)
Zn2++4OH- 
 , β4=1×1014.8 (5)
, β4=1×1014.8 (5)
式中:Ksp为溶度积常数;β为络合物稳定常数。

图6 锌离子组分含量图
Fig. 6 Diagrams of ratio of hydroxyl Zn2+
图6所示为Zn2+的水解组分含量图。当pH<6时,溶液中主要成分存在形式为Zn2+;锌离子在6<pH<8时,主要是以Zn2+和Zn(OH)+以及Zn(OH)2形式存在,Zn(OH)+是优势组分;pH为9~11时,主要是以Zn(OH)2形式和少量Zn(OH)+、Zn(OH)2、 及
及 存在;pH为11~13时,优势组分为
存在;pH为11~13时,优势组分为 ,pH>13时,锌离子优势组分是
,pH>13时,锌离子优势组分是 ;结合图2中矿物可浮性与pH值关系可知,在pH为3~9的范围内油酸钠与异极矿发生相互作用,使异极矿上浮,与油酸钠作用的主要为Zn2+和Zn(OH)+。而异极矿回收率最佳pH值为4~8,则主要是油酸钠与Zn2+和少量Zn(OH)+发生作用,pH为9~11时异极矿的可浮性与图6中Zn(OH)2(aq)(9<pH<11)呈负相关关系,说明Zn(OH)2(surf)过多可能不利于异极矿的浮选,在pH>11时, 水溶液中OH-组分浓度很高,异极矿可浮性的降低可能归因于OH-抑制油酸钠在矿物表面上的吸附。在pH为9~11时异极矿可浮性可能是油酸根离子与矿物表面形成的Zn(OH)2发生离子交换,可用以下方程来表示[27]:
;结合图2中矿物可浮性与pH值关系可知,在pH为3~9的范围内油酸钠与异极矿发生相互作用,使异极矿上浮,与油酸钠作用的主要为Zn2+和Zn(OH)+。而异极矿回收率最佳pH值为4~8,则主要是油酸钠与Zn2+和少量Zn(OH)+发生作用,pH为9~11时异极矿的可浮性与图6中Zn(OH)2(aq)(9<pH<11)呈负相关关系,说明Zn(OH)2(surf)过多可能不利于异极矿的浮选,在pH>11时, 水溶液中OH-组分浓度很高,异极矿可浮性的降低可能归因于OH-抑制油酸钠在矿物表面上的吸附。在pH为9~11时异极矿可浮性可能是油酸根离子与矿物表面形成的Zn(OH)2发生离子交换,可用以下方程来表示[27]:
Zn(OH)2(surf)+RCOO-  Zn+RCOO-+OH- (6)
Zn+RCOO-+OH- (6)
2.2.4 油酸钠溶液化学性质
油酸钠在水溶液中发生水解时所产生的二聚物平衡、胶束等与pH值紧密相关,并且能影响吸附层表面的电性质[28-29]。油酸的溶解度S=1×10-7.6 mol/L,通常浮选用量下,矿浆中油酸浓度均大于溶解度,此时水溶液中溶解的油酸RCOOH(aq)与不溶解的液态油酸RCOOH(l)之间形成饱和溶液,根据文献[27]平衡方程绘出不同油酸钠初始浓度时各组分浓度与pH值的关系。图7所示为3×10-4 mol/L时溶液各组分浓度与pH 值的关系。溶液中RCOOH(l)与RCOOH(aq)平衡时的pH值约为8.55。
在 pH值为4~8 范围内,随着 pH值的增大,油酸钠溶液中RCOO-、 和RCOOH·RCOO-含量上升,异极矿的回收率始终保持在80%左右,油酸钠的优势组分为 RCOOH(aq)和
和RCOOH·RCOO-含量上升,异极矿的回收率始终保持在80%左右,油酸钠的优势组分为 RCOOH(aq)和 。
。 组分的变化趋势和回收率并没有表现出一致,但在pH>3.2时,离子-分子缔合物RCOOH·RCOO-开始形成,而从图2中浮选现象来看,pH值大于3.2时回收率上升较为明显,说明RCOOH·RCOO-存在时可能有助于提高异极矿的回收率。王淀佐等[25]研究认为,离子-分子缔合物的烃链与单个离子相比增大了一倍,在极性基相同的情况下,离子-分子缔合物具有更大的表面活性。因此,除RCOOH(aq)、
组分的变化趋势和回收率并没有表现出一致,但在pH>3.2时,离子-分子缔合物RCOOH·RCOO-开始形成,而从图2中浮选现象来看,pH值大于3.2时回收率上升较为明显,说明RCOOH·RCOO-存在时可能有助于提高异极矿的回收率。王淀佐等[25]研究认为,离子-分子缔合物的烃链与单个离子相比增大了一倍,在极性基相同的情况下,离子-分子缔合物具有更大的表面活性。因此,除RCOOH(aq)、 和RCOO-之外,离子-分子缔合物RCOOH·RCOO-也可能对异极矿的疏水上浮起到一定的决定作用。
和RCOO-之外,离子-分子缔合物RCOOH·RCOO-也可能对异极矿的疏水上浮起到一定的决定作用。
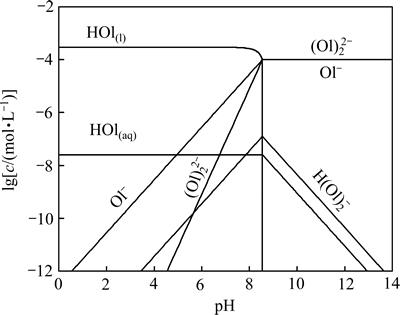
图7 油酸钠溶液组分的lg c-pH图(cT=3×10-4 mol/L)
Fig. 7 Species distribution diagrams of 3×10-4 mol/L sodium oleate as a function of pH
在 pH为弱碱性区域时,油酸钠溶液优势组分为RCOO-和 ,而红外光谱测试结果表明,异极矿表面发生了化学吸附,据此推测应该是矿物表面的锌离子与油酸根离子作用生成了Zn(RCOO)2。此 外,溶液中尚存 RCOOH(aq)和RCOOH·RCOO-,它们之间也可能存在物理吸附。
,而红外光谱测试结果表明,异极矿表面发生了化学吸附,据此推测应该是矿物表面的锌离子与油酸根离子作用生成了Zn(RCOO)2。此 外,溶液中尚存 RCOOH(aq)和RCOOH·RCOO-,它们之间也可能存在物理吸附。
3 结论
1) 油酸钠作用体系下,异极矿在pH为4~8和11时都获得了较好的可浮性,回收率都保持在80%左右。
2) Zeta电位、红外光谱测试结果及锌离子溶解组分含量表明,油酸钠在异极矿表面主要发生了化学吸附,矿物表面的活性点为Zn2+、Zn(OH)+和Zn(OH)2(suf)能与油酸钠发生化学作用生成油酸盐,从而使异极矿疏水上浮。结合油酸钠浮选溶液化学,矿物表面也可能存 RCOOH(aq)和RCOOH·RCOO-的物理吸附,离 子-分子缔合物的存在可能有利于异极矿的上浮。
REFERENCES
[1] GHOSH M K, DAS R P, BISWAS A K. Oxidative ammonia leaching of sphalerit. Part I. Noncatalytic kinetics[J]. Int J Miner Process, 2003, 70(1): 221-234.
[2] SOUZA A D, PINA P S, SANTOS F M F, da SILVA C A, LEAO V A. Effect of iron in zinc silicate concentrate on leaching with sulphuric acid[J]. Hydrometallurgy, 2009, 95(3/4): 207-214.
[3] IRANNAJAD M, EJTEMAEI M, GHARABAGHI M. The effect of reagents on selective flotation of smithsonite-calcite- quartz[J]. Minerals Engineering, 2009, 22(9/10): 766-771.
[4] YUAN Tie-chui, CAO Qin-yuan, LI Jie. Effects of mechanical activation on physicochemical properties and alkaline leaching of hemimorphite[J]. Hydrometallurgy, 2010, 104(2): 136-141.
[5] SAFARI V, ARZPEYMA G, RASHCHI F, MOSTOFI N. A shrinking particle-shrinking core model for leaching of a zinc ore containing silica[J]. Int J Miner Process, 2009, 93(1): 79-83.
[6] 张祥峰, 孙 伟. 阴阳离子混合捕收剂对异极矿的浮选作用及机理[J]. 中国有色金属学报, 2014, 24(2): 499-506.
ZHANG Feng-xiang, SUN Wei. Flotation behaviors and mechanism of hemimorphite in presence of mixed cationic/anionic) collectors[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(2): 499-506.
[7] AMARANTE M M, de SOUSA A B, LEITE M M. Processing a spodumene ore to obtain lithium concentrates for addition to glass and ceramic bodies[J]. Miner Eng, 1999, 12(4): 433-436.
[8] ZHU Y G, ZHANG G F, FENG Q M, YAN D C, WANG W Q. Effect of surface dissolution on flotation separation of fine ilmenite from titanaugite[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1149-1154.
[9] FREE M L, MILLER J D. The significance of collector colloid adsorption phenomena in the fluorite/oleate flotation system as revealed by FTIR/IRS and solution chemistry analysis[J]. Int J Miner, Process,1996, 48(3/4): 197-216.
[10] YIN Wan-zhong, YANG Xiao-sheng, ZHOU Da-peng, LI Yan-jun,  Zhen-fu. Shear hydrophobic flocculation and flotation of ultrafine Anshan hematite using sodium oleate[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 652-644.
Zhen-fu. Shear hydrophobic flocculation and flotation of ultrafine Anshan hematite using sodium oleate[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 652-644.
[11] 张国范, 陈启元, 冯其明, 张平民. 温度对油酸钠在一水硬铝石矿物表面吸附的影响[J]. 中国有色金属学报, 2004, 14(6): 1042-1046.
ZHANG Guo-fan, CHEN Qi-yuan, FENG Qi-ming, ZHANG Ping-min. Influence of temperature on absorption of sodium oleate on surface of diaspore[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 1042-1046.
[12] LU Yong-qiang, DRELICH J, MILLER J D. Oleate adsorption at an apatite surface studied by ex-situ FTIR internal reflection spectroscopy[J]. Journal of Colloid & Interface Science, 1998, 202(2): 462-476.
[13] FAN X, ROWSON N A. The effect of Pb(NO3)2 on ilmenite flotation[J]. Mineral Engineering, 2000, 13(2): 205-215.
[14] SOMASUNDARAN P. 以离子分子络合物为基础的浮选机理[J]. 中南矿冶学院学报, 1983, 8(S2): 59-68.
SOMASUNDARAN P. Flotation mechanism based on ion-molecular complexes[J]. Journal of Central South Institute of Mining and Metallurgy, 1983, 8(S2): 59-68.
[15] HANUMANTHA RAO K, FORSSBERG K S E. Mechanism of fatty acid adsorption in salt-type mineral flotation[J]. Miner Eng, 1991, 4(7/11): 879-890.
[16] SALUM M J G, de ARAUJO A C, PERES A E C. The role of sodium sulphide in amine flotation of silicate minerals[J]. Minerals Engineering, 1992, 5(3/5): 411-419.
[17] PEREIRA C A, PERES A E C. Reagents in calamine zinc ores flotation[J]. Minerals Engineering, 2005, 18(2): 275-277.
[18] PERES A E C, BORGES A A M, GALERY R. The effect of the dispersion degree on the floatability of an oxidased zinc ore[J]. 1994, 7(11): 1435-1439.
[19] MEHDILO A, IRANNAJAD M, REZAI B. Effect of chemical composition and crystal chemistry on the zeta potential of ilmenite[J]. Colloids Surf A: Physicochem Eng Aspects, 2013, 428: 111-119.
[20] FROST R L, BOUZAID J M, JAGANNADHA R B. Vibrational spectroscopy of the sorosilicate mineral hemimorphite Zn4(OH)2Si2O7·H2O[J].Polyhedron, 2007, 26(12): 2405-2412.
[21]  J J. Phase transition behaviour of sodium oleate aerosol particles[J]. Atmos Environ, 2007, 41(5): 1041-1052.
J J. Phase transition behaviour of sodium oleate aerosol particles[J]. Atmos Environ, 2007, 41(5): 1041-1052.
[22] TANDON P, RAUDENKOLB S, NEUBERT R H H, RETTIG W, WARTEWIG S. X-ray diffraction and spectroscopic studies of oleic acid-sodium oleate[J]. Chem Phys Lipids, 2001, 109(1): 37-45.
[23] 冯其明, 赵岩森, 张国范. 油酸钠在赤铁矿及磷灰石表面的吸附机理[J]. 中国有色金属学报, 2012, 22(10): 2902-2907.
FENG Qi-ming, ZHAO Yan-sen, ZHANG Guo-fan. Adsorption mechanisms of sodium oleate on surfaces of hematite and apatite[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2902-2907.
[24] 张国范, 鄢代翠, 朱阳戈, 冯其明, 王维清. pH 对油酸钠在钛铁矿与钛辉石表面吸附的影响[J]. 中国有色金属学报, 2011, 42(10): 2898-2904.
ZHANG Guo-fan, YAN Dai-cui, ZHU Yang-ge, FENG Qi-ming, WANG Wei-qing. Influence of pH on adsorption of sodium oleate on surface of ilmenite and titanaugite[J]. The Chinese Journal of Nonferrous Metals, 2011, 42(10): 2898-2904.
[25] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988.
WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation[M]. Changsha: Hunan Science and Technology Press, 1988.
[26] 王云燕, 柴立元, 王庆伟, 舒余德. 重金属离子(Zn2+, Cu2+, Cd2+, Pb2+)-水系羟合配离子配位平衡研究[J]. 中国有色金属学报, 2008, 18(1): 183-191.
WANG Yun-yan, CHAI Li-yuan, WANG Qing-wei, SHU Yu-de. Thermodynamic equilibrium of hydroxyl complex ions in heavy metals (Zn2+, Cu2+, Cd2+, Pb2+)-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(1): 183-191.
[27] HOSSEINI S H, FORSSBERG E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid[J]. Miner Metall Process SME, 2006, 23: 87-96.
[28] JUNG R F, JAMES R O, HEALY T W. Adsorption, precipitation, and electrokinetic processes in the iron oxide (Goethite)-oleic acid-oleate system[J]. Journal of Colloid & Interface Science, 1987, 118(2): 463-72.
[29] SOMASUNDARAN P, ANANTHAPADMANABHAN K P, IVANOV I B. Dimerization of oleate in aqueous solutions[J]. Journal of Colloid & Interface Science, 1984, 99(1): 128-135.
Flotation behaviors and mechanism of hemimorphite using sodium oleate as collector
LIU Cheng, FENG Qi-ming, ZHANG Guo-fan
(School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The flotation behavior of hemimorphite using sodium oleate as collector was studied through pure mineral test. When the dosage of sodium oleate is 3×10-4 mol/L, and pH of the pulp is set as 4-8, the flotation recovery rate of hemimorphite is all about 80%. Zeta potential and IR spectra results show that the chemical adsorption mainly occurs on the surface of hemimorphite for sodium oleate. Based on the results of solution chemistry calculation of sodium oleate and ratio of hydroxyl Zn2+, RCOO- and (RCOO)22- are the main compositions when the pH of the pulp is from 6 to 8, Zn2+ and ZnOH+ are the major components of the hydroxyl compound of Zn2+ on the surface of hemimorphite. Combined with the results of hemimorphite flotation behavior, the surface interaction mechanism of hemimorphite with sodium oleate is as follows: Zn2+ and ZnOH+ on hemimorphite surface are activated adsorption sites and able to chemically react with sodium oleate. Besides, physical adsorption of RCOOH(aq) and RCOOH·RCOO- on rutile may also happen. The flotation recovery of hemimorphite around pH of 11 may be attributed to interaction between RCOO- and zinc on the hemimorphite surface and can be assumed that the adsorption of sodium oleate takes place by an ion exchange mechanism.
Key words:hemimorphite; sodium oleate; flotation
Foundation item: Project(2014CB643402) supported by the National Basic Research Development Program of China
Received date: 2015-07-09; Accepted date: 2015-10-26
Corresponding author: ZHANG Guo-fan; Tel: +86-731-8830913; E-mail: zhangguofan204@sina.com
(编辑 王 超)
基金项目:国家重点基础研究发展计划资助项目(2014CB643402)
收稿日期:2015-07-09;修订日期:2015-10-26
通信作者:张国范,教授,博士;电话:0731-8830913;E-mail: zhangguofan204@sina.com