J. Cent. South Univ. (2014) 21: 1285-1289
DOI: 10.1007/s11771-014-2064-7

Preparation and electrochemical performance of Li2Mn0.5Fe0.5SiO4 cathode material with sol-gel method for lithium ion batteries
HU Chuan-yue(胡传跃), GUO Jun(郭军), WEN Jin(文瑾), PENG Yang-xi(彭秧锡)
Department of Chemistry and Material Science, Hunan Institute of Humanities, Science and Technology,Loudi 417000, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2014
Central South University Press and Springer-Verlag Berlin Heidelberg 2014
Abstract: Li2Fe0.5Mn0.5SiO4 material was synthesized by a citric acid-assisted sol-gel method. The influence of the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) on the electrochemical properties of Li2Fe0.5Mn0.5SiO4 was studied. The final sample was identified as Li2Fe0.5Mn0.5SiO4 with a Pmn21 monoclinic structure by X-ray diffraction analysis. The crystal phases components and crystal phase structure of the Li2Fe0.5Mn0.4SiO4 material were improved as the increase of the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+). Field-emission scanning electron microscopy verified that the Li2Fe0.5Mn0.5SiO4 particles are agglomerates of Li2Fe0.5Mn0.5SiO4 primary particles with a geometric mean diameter of 220 nm. The Li2Fe0.5Mn0.5SiO4 sample was used as an electrode material for rechargeable lithium ion batteries, and the electrochemical measurements were carried out at room temperature. The Li2Fe0.5Mn0.5SiO4 electrode delivered a first discharge capacity of 230.1 mAh/g at the current density of 10 mA/g in first cycle and about 162 mAh/g after 20 cycles at the current density of 20 mA/g.
Key words: lithium ion battery; Li2Fe0.5Mn0.5SiO4; citric acid assisted sol-gel method; cathode
1 Introduction
Rechargeable lithium ion batteries are considered to be one of the most advanced energy storage systems used by today’s information-rich and mobile society. Now, commercial lithium-ion batteries mostly rely on conventional lithium transition metal oxide as cathode materials [1-6]. However, the toxicity, safety problem and high cost of cobalt represent some of the problems of the transition metal oxidation. Recently, poly anion compounds with (XO4)n- group (X=P, Si, Ge, and etc.) have been extensively investigated owing to their safety and lower cost, in spite of their intrinsic low electronic conductivity and slow lithium ion diffusion [7-9]. The poly anion-type cathode materials have better safety characteristics compared with the lithium metal oxide materials, which may act as strong oxidizers at a highly charged state while contacting with an organic electrolyte [7]. The theoretical capacity of Li2MSiO4 as poly anionic cathodes can reach as high as 330 mAh/g (e.g. 333 mAh/g for M=Mn; 325 mAh/g for M=Co; and 325.5 mAh/g for M=Ni) [11]. The theoretical capacity of LiMPO4 is 170 mAh/g, where usually only one electron exchange is available.
NYTEN et al [12] first reported Li2FeSiO4 as a new cathode material. The Li2FeSiO4 material showed a reversible capacity of around 130 mAh/g in the first cycle at a rate of C/16. As Li2FeSiO4 operates on only one-electron redox couples, Fe3+/Fe2+, its theoretical and practical capacity is limited. The theoretical capacity of Li2FeSiO4 material is 166 mAh/g.
Recently, DOMINKO et al [13] reported some results about Li2MnSiO4 as a cathode material. About 0.6 Li was reversibly exchanged in the first cycle, but the reversible capacity faded considerably to only about 0.3 Li in the fifth cycle at a rate of C/30 ratio in their work. This poor cycling performance resulted from the change of initial structure of Li2MnSiO4 during the first charge-discharge process. Compared to Li2MnSiO4 [13-14] and Li2CoSiO4 [15-17], Li2FeSiO4 has been attracted much more attention due to the high cycling stability [18]. Coating carbon on the surface of Li2FeSiO4 particles was an effected method to improve the poor electron conductivity [19-21]. However, the poor electron conductivity of Li2MSiO4 can be improved by using cation doping which has positive effect on the structural and electrochemical properties of Li2FeSiO4. According to the similar radius, the Fe2+ in Li2FeSiO4 material can be substituted with Cu2+, Ni2+ and Zn2+ [22]. The initial structure of Li2FeSiO4 can be stabilized by doping Mn2+ to prepare Li2MnxFe1-xSiO4 [24]. In fact,the Li2MnxFe1-xSiO4 has been successfully synthesized and the Li2Mn0.5Fe0.5SiO4 was found to be able to deliver excellent electrochemical performance [20-21]. A reversible capacity of 214 mAh/g was achieved for Li2Fe0.5Mn0.5SiO4 material with sucrose as carbon resources [20]. On the other hand, the poor electron counductivity was a key element to result poor electrochemical performance of silicate. In order to impove the electrochemical properties of the LiFePO4 material, two methods were used to prepare the nano-particle to shorten the transfer distance of lithium ion and coating carbon on the LiFePO4 particle [25].
In this work, we investigated the impact of citric acid on the crystal structural and electrochemical characteristics of Li2Fe0.5Mn0.5SiO4. All the samples were prepared by a citric acid-assisted sol-gel method.
2 Experimental
The Li2Fe0.5Mn0.4SiO4 precursor was prepared by a sol-gel method. Firstly, the stoichiometric amount of LiAC·2H2O, Mn(AC)2·4H2O and citric acid was dissolved into water. Then, a designed amount of (C2H5)4SiO4 ethonal solution and FeC2O4·2H2O were added into the mixture solution. The mixture was stirred at 60 °C for 24 h in a reflux system and then dried at 110 °C in vacuum after ethonal-water solvents were evaporated by reduced pressure distillation. Lastly, the mixture was pressed into pellets after milling for 10 h. The prepared precursors were heated to 700 °C for 10 h in a flow of Ar.
Morphology of the fabricated sample was characterized by field emission scanning electron microscopy (SEM, FEI Quanta-200) operated at an acceleration voltage of 5 kV. Powder X-ray diffraction (XRD, Philips X’Pert MPD diffractometer, Cu Kα radiation) was used for phase identification. Scan rate of 4(°)/min and step size of 0.04° were applied to record the pattern. The operation voltage and current were 40 kV and 45 mA, respectively.
The electrochemical performance of the Li2Fe0.5Mn0.4SiO4/C as the positive electrode was evaluated using a coin-type cell (size 2025) with a lithium metal anode. The working electrode was produced by dispersing 90% (mass fraction) active materials, 3% (mass fraction) carbon black, and 7% (mass fraction) polyvinylidene fluoride (PVDF) binder in N-methylpyrrolidone (NMP) solvent to form a homogeneous slurry. The slurry was then spread on Al foil. The coated electrode was dried in vacuum at 120 °C for 12 h. The working electrode comprised approximately 5 mg of the active mass. The electrolyte was 1 mol/L LiPF6 in a mixture of ethylene carbonate (EC)-dimethylene carbonate (DMC)-ethylmethyl carbonate (EMC) (1:1:1 in volume). Galvanostatic charge-discharge cycling was carried out with a multichannel battery tester (BK6061 testing system, Guangzhou Lanqi Electronic Co.Ltd., China). A constant current-constant voltage (CC-CV) protocol was used with the potential range of 1.5-4.8 V versus Li/Li+ at a current density of 10 mA/g at 25°C in the first cycle and at a current density of 20 mA/g for the cycling life test. The constant voltage was applied until the charging current dropped to values corresponding to a 0.02 mA.
3 Results and discussion
The XRD patterns of the Li2Fe0.5Mn0.5SiO4 powder are shown in Fig. 1. The results of X-ray diffraction show that the crystal system of Li2Fe0.5Mn0.4SiO4/C solid solution is identified with a Pmn21 monoclinic structure, which is similar to the iso-structural of low-temperature Li3PO4 [12, 26]. The most intense diffraction peak of Li2Fe0.5Mn0.4SiO4/C samples is observed at 24°, which is similar with the most intense diffraction peak of LiMnSiO4/C and Li2CoSiO4/C [13,16]. The impurities of Fe2O3 and FeO are detected in the samples prepared when the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) was from 0.2 to 0.4. The peak intensity of impurities decreased with the increase of stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+). The Fe2O3 purity resulted from the oxidation of FeC2O4 was reduced to Fe2+ by the carbon thermal reduction reaction with the increase of stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+). The good crystal structure of Li2Fe0.5Mn0.4SiO4 material with high (022) peak intensity was obtained when the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) is 0.4 and 0.5 . Therefore, the crystal phases components and crystal phase structure for the synthesized Li2Fe0.5Mn0.4SiO4 material were strongly influenced by the stoichiometric ratio value of n (citric acid) to n (Fe2+-Mn2+).
The particle morphology of the Li2Fe0.5Mn0.5SiO4
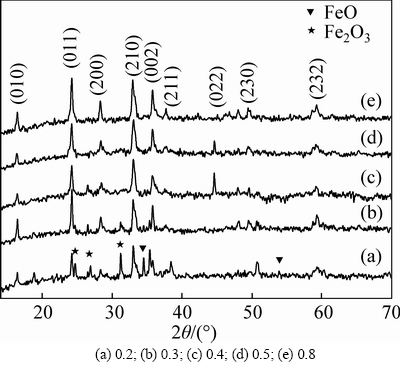
Fig. 1 XRD patterns of Li2Fe0.5Mn0.5SiO4 with different stoichiometric ratio values of n(citric acid) to n(Fe2+-Mn2+):
sample prepared with stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+)=0.5 was observed by scanning electron microscopy (SEM), and the image is shown in Fig. 2. SEM analysis indicates that Li2Fe0.5Mn0.5SiO4 powder consists of agglomerates of primary particles. The average size of primary particles is about 221 nm, which means the shorter diffusion distance of lithium ion and improves the electrochemical performance of Li2Fe0.5Mn0.5SiO4 materials with poor electron conductivity. The particle shape is approximately spherical. The spherical particles are resulted from the addition of carbon, which inhibit the particle growth and induce the formation of spherical particle during sintering process. On the other hand, the carbon coming from the citric acid coated on the surface of Li2Fe0.5Mn0.5SiO4 particle and improved the electronic conductivity.
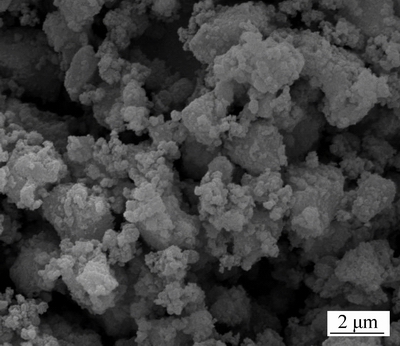
Fig. 2 SEM image of Li2Fe0.5Mn0.5SiO4 powder (Stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) is 0.5)
The electrochemical performances of Li2Fe0.5Mn0.5SiO4 were investigated and the influence of citric acid content on the electrochemical performance was tested. Figure 3 shows the initial voltage profiles of Li2Fe0.5Mn0.5SiO4 samples in a voltage range of 1.5-4.8 V vs Li/Li+ at a current density of 10 mA/g. The discharge capacity and coulombic efficiency are listed in Table 1. The initial voltage profile shows two voltage plateaus at 4.2 V and 4.4 V during the charge process and at 3.3 V and 2.9 V during the sequent discharge process. When the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) is 0.5, the Li2Fe0.5Mn0.5SiO4 shows the excellent electrochemical performance. The initial reversible capacity is 220.4 mAh/g, which is the 88.3% of the theoretic capacity in the first cycle. The coulombic efficiency is 88.3%. The theoretic capacity of Li2Fe0.5Mn0.5SiO4 is 249.1 mAh/g according to the following equation:

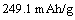 (1)
(1)
In addition, the reversible capacity of Li2Fe0.5Mn0.5SiO4 increases with the increase of stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) from 0.2 to 0.5, which indicates that the poor electron conductivity of Li2Fe0.5Mn0.5SiO4 is improved by the coated carbon on the particle surface.
Figure 4 shows the differential capacity (dQ/dV)
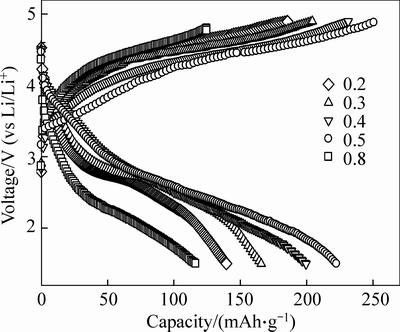
Fig. 3 Initial charge-discharge curves of Li2Fe0.5Mn0.5SiO4 material with different stoichiometric ratio values of n(citric acid) to n(Fe2+-Mn2+)
Table 1 Charge-discharge data of Li2Fe0.5Mn0.5SiO4 in initial charge-discharge cycle with different stoichiometric ratio values of n(citric acid) to n(Fe2+-Mn2+)
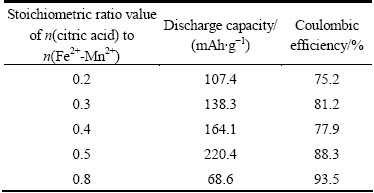
Fig. 4 Differential capacity plots of Li2Fe0.5Mn0.5SiO4 sample during first cycle (Stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) is 0.5)
plots for the Li2Fe0.5Mn0.5SiO4/C sample during the first cycle. Four oxidation peaks are observed at the potentials of 3.5, 4.10, 4.25 and 4.48 V, corresponding to the oxidation reactions of SEI film formation, Fe2+→Fe3+, Mn2+→Mn3+ and Mn3+→Mn4+. The four reduction peaks are also observed at the potentials of 3.70, 2.70, 2.35 and 1.90 V, which correspond to the reduction reactions of Fe3+→Fe2+, Mn4+→Mn3+, Mn3+→Mn2+ and SEI film formation. These results indicate that the second lithium ion in per unit formula of Li2Fe0.5Mn0.5SiO4/C is reversible extracted and inserted in the voltage range of 1.5-4.8 V vs Li/Li+.
EIS measurements are conducted from 0.01 Hz to 100 kHz. The Nyquist plots of the Li2Fe0.5Mn0.5SiO4 electrodes after the first cycle are shown in Fig. 5. The Nyquist plots exhibit the similar curves consisting of a depressed semicircle in high-medium frequency and a 45° line in low frequency. It is supposed that the high-medium frequency semicircle corresponds to the charge-transfer resistance rather than the intrinsic resistance and contact resistance. As can be seen from Fig. 5, the internal resistances (Rs) of the electrode materials are 1605, 1423, 798, 580 and 1600 W when the stoichiometric ratio value n(citric acid) to n(Fe2+-Mn2+) is 0.2, 0.3, 0.4, 0.5 and 0.8. Rs includes the ionic resistance of electrolyte (Re), the intrinsic resistance of the active material (Ra) and the contact resistance (Ri) at the interface active material/ current collector. The results showed that the AC impedance of Li2Fe0.5Mn0.5SiO4 was obviously influenced by the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+), which suggested that the electronic conduction of Li2Fe0.5Mn0.5SiO4 was strongly influenced. When the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) is 0.5, the Li2Fe0.5Mn0.5SiO4 electrodes show the lower internal resistances, which indicates that the lithium ions and electrons can transfer easily across the Li2Fe0.5Mn0.5SiO4/ electrolyte interfaces, leading to the enhanced electrode reaction kinetics and the

Fig. 5 Nyquist plots of Li2Fe0.5Mn0.5SiO4 electrodes with different stoichiometric ratio values of n(citric acid) to n(Fe2+-Mn2+) after first cycle
improved electrochemical performance.
Figure 6 shows the cycling performance of the Li2Fe0.5Mn0.5SiO4 samples at a current density of 20 mA/g. Clearly, the discharge capacity of all samples fades rapidly from the first cycle to the 5th cycle and gradually stabilizes after 10 cycles. When the stoichiometric ratio value of n(citric acid) : n(Fe2+-Mn2+) is 0.5, Li2Fe0.5Mn0.5SiO4 cathode shows good cycling performance, and discharge capacity is stabilized at 162 mAh/g after 20 cycles. The electrochemcial performance of Li2Fe0.5Mn0.5SiO4 was influenced by the stoichiometric ratio value of n (citric acid) : n (Fe2+-Mn2+) due to the different complex effect between citric acid and transition metal ions. The Fe2O3 purity could be produced by the oxidation of FeC2O4, which can decrease the electrochemical performance of Li2Fe0.5Mn0.5SiO4 materials. However, the Fe3+ can be reduced to the Fe2+ by the carbon thermal reduction reaction when the increase of stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) increased from 0.2 to 0.5. The electrochemical perfomance of Li2Fe0.5Mn0.5SiO4 can be improved by increasing the n value.
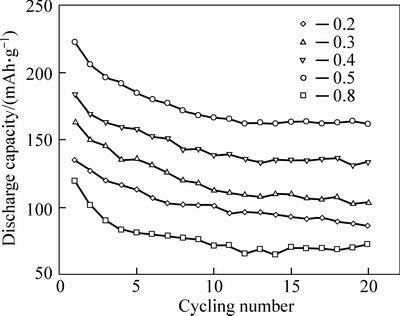
Fig. 6 Cycling performance of Li2Fe0.5Mn0.5SiO4 electrodes with different stoichiometric ratio values of n(citric acid) to n(Fe2+-Mn2+) at current density of 20 mA/g
4 Conclusions
1) Li2Fe0.5Mn0.5SiO4 positive-electrode material for lithium-ion batteries is synthesized by using a citric acid assisted sol-gel method. The influence of stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+) on the electrochemical properties of Li2Fe0.5Mn0.5SiO4 materitals is investigated. Li2Fe0.5Mn0.5SiO4 solid solution is identified with a Pmn21 monoclinic structure by XRD analysis.
2) The electrochemical performance of Li2Fe0.5Mn0.5SiO4 samples are greatly influenced by the stoichiometric ratio value of n(citric acid) to n(Fe2+-Mn2+). When the stoichiometric ratio of n(citric acid) to n(Fe2+-Mn2+) is 0.5, the Li2Fe0.5Mn0.5SiO4 material shows the higher reversible capacity and good cycling performance. The discharge capacity is 230.1 mAh/g in the first cycle at the current densities of 10 mA/g and 162 mAh/g after 20 cycles at the current density of 20 mA/g. Three oxidation-reduction reactions occur between the Fe2+/Fe3+, Mn2+/Mn3+ and Mn3+/Mn4+ redox couples according to the the differential capacity (dQ/dV) plots.
3) Field-emission scanning electron microscopy verified that the Li2Fe0.5Mn0.5SiO4 composites are agglomerates of Li2Fe0.5Mn0.5SiO4 primary particles with a geometric mean diameter of 221 nm, indicating the shorter diffusion distance of lithium ion.
4) The electrochemical performance of Li2Fe0.5Mn0.5SiO4 will be improved by doping transination metal ions to substitute to the Fe2+ and Mn2+ and by doping Al3+ to substitute the Si4+.
References
[1] MAKIMUR A, TANAKA I, ADACHI H, OGZUKU T. Systematic research on insertion materials based on superlattice models in a phase triangle of LiCoO2-LiNiO2-LiMnO2 I. first-principles calculation on electronic and crystal structures, phase stability and new LiNi1/2Mn1/2O2 material [J]. J Electrochem Soc, 2004, 151(9): A1499-A1506.
[2] CHANG Zhao-rong, CHEN Zhong-jun, WU Feng, TANG Hong-wei, ZHU Zhi-hong, YUAN Xiao-zi, WANG Hai-jiang. Synthesis and characterization of high-density non-spherical Li(Ni1/3Co1/3Mn1/3)O2 cathode material for lithium ion batteries by two-step drying method [J]. Electrochim Acta, 2008, 53: 5927-5933.
[3] CHUNG Kyung-yoon, KIM Kwang-bum. Investigations into capacity fading as a result of a Jahn-Teller distortion in 4 V LiMn2O4 thin film electrodes [J]. Electrochim Acta, 2004, 4(20): 3327-3337.
[4] WU Yong-min, WEN Zhen-hai, FENG Hong-bin, LI Jing-hong. Hollow porous LiMn2O4 microcubes as rechargeable lithium battery cathode with high electrochemical performance [J]. Small, 2012, 8(6): 858-862.
[5] YANG Yan, LIANG Qing-qin, LI Jing-hong, ZHUANG Yuan, HE Yun-hua, BAI Bo, WANG Xun. Ni3Si2O5(OH)4 multi-walled nanotubes with tunable magnetic properties and their application as anode materials for lithium batteries [J]. Nano Res, 2011, 4(9): 882-890.
[6] WEN Z, WANG Q, ZHANG Q, LI J. In situ growth of mesoporous SnO2 on multiwalled carbon nanotubes: A novel composite with porous-tube structure as anode for lithium batteries [J]. Adv Funct Mater, 2007, 17(15): 2772-2778.
[7] PAKHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J].J Electrochem Soc, 1997, 144: 1188-1194.
[8] AMINE K, YASUDA H, YAMACHI M. Olivine LiCoPO4 as 4.8 V electrode material for lithium batteries [J]. Electrochem Solid-State Lett, 2000, 3: 178-179.
[9] LI Guo-hua, HIDTO Azuma, MASAYUKI Tohda, LiMnPO4 as the cathode for lithium batteries [J].Electrochem Solid-State Lett, 2002, 5: A135-A137.
[10] ZHANG Z R, GONG Z L, YANG Yong. Electrochemical performance and surface properties of bare and TiO2-Coated cathode materials in lithium-ion batteries [J]. J Phys Chem B 2004, 108: 17546-17552.
[11] LI Yi-xiao, GONG Zheng-liang, YANG Yong. Synthesis and characterization of Li2MnSiO4/C nanocomposite cathode material for lithium ion batteries [J].J Power Sources, 2007, 174: 528-532.
[12] NYTEN A, ABOUIMRANE A, ARMAND M, GUSTAFFSON T, THOMAS J O. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material [J]. Electrochem Commun, 2005, 7: 156-160.
[13] DOMINKO R, BELE M, GABERSCEK M, MEDN A, REMSKAR M, JAMNIK J. Structure and electrochemical performance of Li2MnSiO4 and Li2FeSiO4 as potential Li-battery cathode materials [J].Electrochem Commun, 2006, 8: 217-222.
[14] ARAVINDAN V, RAVI S, KIM W S, LEE S Y, LEE Y S. Size controlled synthesis of Li2MnSiO4 nanoparticles: Effect of calcinations temperature and carbon content for high performance lithium batteries [J]. J Colloid and Interface Sicence, 2011, 355: 472-477.
[15] NESS C, DELOBEL B, ARMSTRONG A R, BRUCE P G. The lithium intercalation compound Li2CoSiO4 and its behaviour as a positive electrode for lithium batteries [J]. Chem Commun (Camb) 2007, 46: 4890-4892.
[16] GONG Zheng-liang, LI Yi-xiao, YONG Yong. Synthesis and electrochemical performance of Li2CoSiO4 as cathode material for lithium ion batteries [J]. J Power Sources, 2007, 174: 524-527.
[17] WU Shun-qing, ZHU Zi-zhong,YANG Yong, HOU Zhu-feng, Effects of Na-substitution on structural and electronic properties of Li2CoSiO4 cathode material [J].Trans Nonferrous Met Soc China, 2009, 19: 182-186.
[18] PENG Zhong-dong, CAO Yan-bing, HU Guo-rong, DU Ke, GAO Xu-guang, XIAO Zheng-wei. Microwave synthesis of Li2FeSiO4 cathode materials for lithium-ion batteries [J]. Chinese Chemical Letters, 2009, 20: 1000-1004.
[19] GUO Hua-jun, XIANG Kai-xiong, CAO Xuan, LI Xin-hai, WANG Zhi-xing, LI Li-ming. Preparation and characteristics of Li2FeSiO4/C composite for cathode of lithium ion batteries [J]. Trans Nonferrous Met Soc China, 2009, 19: 166-169.
[20] ZHENG Zongmin, WANG Yan, ZHANG, Ai, ZHANG Tianran, CHENG Fangyi, TAO Zhanliang, CHEN Jun. Porous Li2FeSiO4/C nanocoposite as the cathode material of lithium-ion batteries [J]. J Power Sources, 2012, 198: 229-235.
[21] KINSON C KAM, TORBJORN GUSTAFSSON, JOHN O THOMAS. Synthesis and electrochemical properties of nanostructured Li2FeSiO4/C cathode material for Li-ion batteries [J]. Solid State Ionics, 2011, 192: 356-359.
[22] DENG C, ZHANG S, YANG S Y, FU B L, MA L. Synthesis and characterization of Li2Fe0.97M0.03SiO4 (M=Zn2+, Cu2+, Ni2+) cathode materials for lithium ion batteries [J]. J Power Sources, 2011, 196: 386-392.
[23] GONG Zheng-liang, LI Yi-xiao, YONG Yong. Synthesis and characterization of Li2MnxFe1–xSiO4 as a cathode material for lithium-ion batteries [J]. Electrochem Solid-State Letters, 2006, 9: A542-A544.
[24] ANTON KOKALJ, ROBERT DOMINKO, GREGOR MALI, ANTON MEDEN, MIRAN GABERSCEK, JANEZ JAMNIK. Beyond one-electron reaction in Li cathode materials:  Designing Li2MnxFe1-xSiO4 [J]. Chem Mater, 2007, 19: 3633-3640.
Designing Li2MnxFe1-xSiO4 [J]. Chem Mater, 2007, 19: 3633-3640.
[25] WU Yong-min, WEN Zhen-hai, LI Jing-hong. Hierarchical Carbon-Coated LiFePO4 nanoplate microspheres with high electrochemical performance for Li-ion batteries [J]. Adv Mater, 2011, 23(9): 1126-1129
[26] ZAGHIB K, AIT SALAH A, RAVET N, MAUGER A, GENDRON F, JIOEN C M. Structural, magnetic and electrochemical properties of lithium iron orthosilicate [J]. J Power Sources, 2006, 160: 1381-1386.
(Edited by DENG Lü-xiang)
Foundation item: Projects(13A047, 10B054) supported by the Scientific Research Fund of Hunan Provincial Education Department, China; Projects (2011GK2002, 2011FJ3160) supported by the Planned Science and Technology Project of Hunan Province, China
Received date: 2012-11-23; Accepted date: 2013-12-12
Corresponding author: HU Chuan-yue, PhD; Tel: +86-738-8325065; E-mail: huchuanyue@vip.sina.com.cn