臭氧氧化降解吡虫啉的动力学研究
王希诚1, 2,高乃云1,冯冬梅1,邓扬3,周超1, 4
(1. 同济大学 污染控制与资源化研究国家重点实验室,上海,200092;
2. 华东建筑设计研究院有限公司,上海,200002;
3. 蒙特克莱尔州立大学 地球与环境学院,新泽西,07043;
4. 上海市政工程设计研究总院(集团)有限公司,上海,200092)
摘要:研究臭氧氧化工艺降解吡虫啉的动力学特性以及臭氧分子和羟基自由基分别与吡虫啉反应的动力学特性。采用竞争动力学法,以对氯苯甲酸(pCBA)为竞争参照物测定吡虫啉与羟基自由基的二级反应速率常数(k·OH-Imid)。研究结果表明:臭氧可以有效降解水中的吡虫啉;在臭氧平均质量浓度为1.15 mg/L时,随着pH由6.0增至8.6,准一级动力学常数由0.079 min-1增至0.326 min-1;在pH为7.0条件下,臭氧平均质量浓度为1.41 mg/L,当碱度(以CaCO3计)由0 mg/L增至250 mg/L时,吡虫啉降解的准一级动力学常数由0.121 min-1降至0.034 min-1。在pH为2.0及50 mmol/L叔丁醇存在的条件下,臭氧分子与吡虫啉反应的二级动力学常数为(10.92±0.12) (mol/L)-1·s-1;当pH为7.0时,吡虫啉与羟基自由基反应的二级动力学常数为2.92×109 (mol/L)-1·s-1;当pH在6.0~8.6范围内变化时,此二级反应动力学常数在2.65×109~3.79×109 (mol/L)-1·s-1范围内波动。
关键词:吡虫啉;臭氧;羟基自由基;动力学
中图分类号:X592 文献标志码:A 文章编号:1672-7207(2014)10-3712-07
Kinetics study of imidacloprid degradation by ozonation
WANG Xicheng1, 2, GAO Naiyun1, FENG Dongmei1, DENG Yang3, ZHOU Chao1, 4
(1. State Key Laboratory of Pollution Control and Resource Reuse, Tongji University, Shanghai 200092, China;
2. East China Agrchitectural Design & Research Institute Co. Ltd, Shanghai 200002, China;
3. Department of Earth and Environmental Studies, Montclair State University, NJ 07043, USA;
4. Shanghai Municipal Engineering Design Institute (Group) Co. Ltd, Shanghai 200092, China)
Abstract: Kinetics tests in ozonation of imidacloprid in water were performed. The kinetics for reactions between imidacloprid with molecular ozone and hydroxyl radicals were investigated. An indirect competition method was used to determine the second-orderrate constant for hydroxyl radicaloxidationof imidacloprid (k·OH-Imid) in the presence of p-chlorobenzoic acid(pCBA) as the reference compound. The results demonstrate that the ozonation is an effective method to remove imidacloprid from water. The pseudo first-order rate constants of imidacloprid degradation increases from 0.079 min-1 to 0.326 min-1 with the increase of pH from 6.0 to 8.7 at an average ozone dose of 1.15 mg/L. When the alkalinity (CaCO3) increases from 0 mg/L to 250 mg/L at pH 7.0 and an average ozone dose of 1.41 mg/L, the pseudo first order rate constant decreases from 0.121 to 0.034 min-1. The second-order rate constant ((10.92±0.12) (mol/L)-1·s-1 for the reaction of imidacloprid and molecular ozone is determined at pH 2.0 and in the presence of 50 mmol/L tert-butyl alcohol. The rate constant is 2.92×109 (mol/L)-1·s-1 at pH 7.0. The rate constants are estimated to range at 2.65×109-3.79×109 (mol/L)-1·s-1 at pH 6.0-8.6.
Key words: Imidacloprid; ozone; hydroxyl radicals; kinetics
吡虫啉(英文名Imidacloprid),是广泛应用于农业生产中的硝基亚甲基类内吸杀虫剂,可有效防治水稻、棉花、水果、蔬菜等多种作物上的害虫[1],以及哺乳动物如狗、猫等身上的跳蚤等[2]。从1996年开始,吡虫啉的生产和使用一直呈现快速上升的趋势[3]。目前,吡虫啉已经是全球市场上销量最大的杀虫剂品种[4]。在中性和酸性条件下,吡虫啉可以稳定存在于水中,这就导致了其渗入地下从而污染地下水源的风险,因此,吡虫啉被列为一级危险药品[5];急性暴露于这种杀虫剂可能会导致痉挛、甲状腺损伤,甚至影响哺乳动物的繁殖[5]。近年来,吡虫啉受到了水处理领域专家学者的广泛关注,他们分别研究了光降解[6-8]、生物降解[9]、Fenton氧化[5, 10-11]、吸附[12-13]等工艺对吡虫啉的去除效果。然而,关于臭氧氧化工艺降解水中吡虫啉的研究尤其是动力学研究却比较少。作为一种常用的水处理工艺,臭氧氧化可以有效去除水中的多种有机微污染物如药物[14]、内分泌干扰物[15]、农药[16-17]、抗生素[18]等。臭氧氧化目标有机物的机理包括由臭氧分子引发的直接氧化过程和由臭氧自分解产生的羟基自由基引发的间接氧化过程;臭氧氧化降解有机物的效果还会受到pH、水温、共存粒子等很多因素的影响[19]。本文作者主要研究水质条件pH和碱度对臭氧降解吡虫啉效果的影响,探索臭氧氧化吡虫啉的直接氧化过程和间接氧化过程的二级动力学特性,并对吡虫啉在臭氧氧化工艺中的降解机理进行分析,以期为臭氧氧化吡虫啉的实际应用提供参考。
1 实验材料与方法
1.1 实验试剂与材料
吡虫啉(纯度>99%)和对氯苯甲酸(纯度>99%)均购自西格玛奥德里奇集团(Sigma-Aldrich),其他试剂包括乙腈、甲醇、亚硫酸钠、过氧化氢、碘化钾、叔丁醇(分析纯)和二磺酸靛蓝(纯度>90%),购自中国国药集团化学试剂有限公司。
试验中所用的溶液均用超纯水(电阻率≥18.2 MΩ·cm)配制,超纯水产自Milli-Q水净化仪(XY- 211A)。
1.2 实验方法
实验采用半连续式反应器,如图1所示。实验基本步骤为:先在反应器中加入1000 mL 超纯水(必要时加入适量磷酸盐缓冲液或NaHCO3以调节反应溶液的pH或碱度),先用臭氧发生器(哈尔滨久久电化学科技有限公司,DHX-IX)持续通入臭氧化空气5 min,此时水溶液中的臭氧已至饱和,然后加入吡虫啉以启动实验,并开始计时,在实验过程中仍持续通入臭氧化空气以维持恒定的臭氧浓度。按照一定的时间间隔取样,将20 mL样品加入到二磺酸靛蓝溶液中以测定臭氧含量;另取1 mL样品加入液相进样瓶中,用0.02 mL浓度为0.1 mol/L的亚硫酸钠溶液终止氧化反应以测定吡虫啉残留量。实验过程中的臭氧尾气用质量分数为2%的碘化钾溶液吸收。实验温度为(18±1) ℃。
在测定臭氧分子与吡虫啉的二级反应动力学常数时,反应条件为pH=2.0,加入50 mmol/L叔丁醇。在较低的pH条件下,产生的羟基自由基很少,且叔丁醇是很好的羟基自由基捕获剂,因此,在此实验过程中可以认为臭氧是主要的氧化剂。
在测定羟基自由基与吡虫啉的二级反应动力学常数时,采用竞争动力学方法,以对氯苯甲酸(pCBA)为参照物,在实验开始时加入2.0×10-6 mol/L的过氧化氢(H2O2与O3的物质的量比为0.5[20]),然后,同时加入等浓度的吡虫啉和对氯苯甲酸。吡虫啉和对氯苯甲酸的初始浓度均为6.0×10-6 mol/L。
1.3 分析方法
吡虫啉和对氯苯甲酸的浓度用高效液相色谱仪(岛津,LC-2010A HT)测定,色谱柱为VP-ODS C18反相色谱柱(5 μm×250 mm×4.6 mm);吡虫啉的检测条件为:流动相中φ(乙腈):φ(水)=50%:50%,等梯度洗脱,流动相流速为1.0 mL/min,检测波长为254 nm。对氯苯甲酸的检测条件为流动相—甲醇:摩尔浓度为10 mmol/L磷酸水溶液(φ(磷酸):φ(水)=60%:40%),等梯度洗脱,流动相流速为1.0 mL/min,检测波长为240 nm。臭氧浓度采用靛红钾法[21],利用紫外分光光度计(UV-4802H, 尤尼柯)进行检测,检测波长为610 nm。pH采用雷磁PHS-3C精密pH计测定。
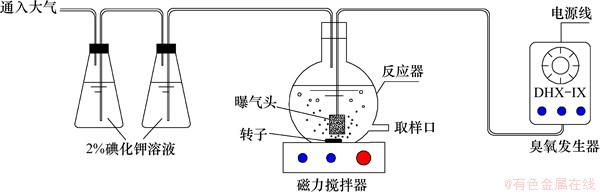
图1 臭氧氧化吡虫啉的实验装置
Fig. 1 Experimental device for imidcloprid ozonation
2 实验结果与讨论
2.1 中性条件下吡虫啉在臭氧氧化过程中的降解效果研究
在中性条件下,设计了3组平行实验,吡虫啉初始质量浓度为0.5 mg/L,实验结果如图2所示。从图2可以看出:在每一组实验过程中,臭氧的含量均保持恒定,在臭氧平均质量浓度为1.35 mg/L时,30 min内,吡虫啉的降解率可以达到96%以上。
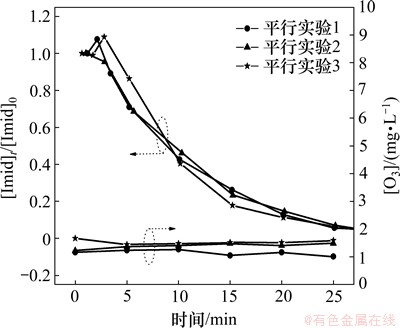
图2 中性条件下臭氧氧化吡虫啉的效果及实验过程中臭氧浓度的变化曲线
Fig. 2 Degradation of imidacloprid by ozonation at neutral conditions and concentration levels of ozone during each set of experiment
在臭氧氧化过程中,臭氧可以通过反应(1)和反应(2)产生羟基自由基[22]。
O3+OH-→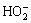 +O2,k1=70 (mol/L)-1·s-1 (1)
+O2,k1=70 (mol/L)-1·s-1 (1)
O3+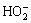 →
→ OH+
OH+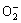 +O2,k2=2.8×106 (mol/L)-1·s-1 (2)
+O2,k2=2.8×106 (mol/L)-1·s-1 (2)
吡虫啉分别与臭氧分子和羟基自由基的初级反应可以表达为
O3+ Imidacloprid→by-product A (3)
·OH+Imidacloprid→by-product B (4)
因此,吡虫啉的总降解速率可以表达为
-d[Imd]t/dt=kO3-Imid[Imid]t[O3]t+k·OH-Imid[Imid]t[·OH]t (5)
式中:kO3-Imid和k·OH-Imid分别为吡虫啉与臭氧分子和羟基自由基反应的二级速率常数,(mol/L)-1·s-1;[Imid]t,[O3]t和[·OH]t分别为t时刻吡虫啉、臭氧和羟基自由基的浓度,mol/L;t为反应时间,s。
在每一组实验中,[O3]t保持恒定,如图2所示;根据Rct理论[23],在臭氧质量浓度恒定且水质不变的条件下,水中羟基自由基的含量也恒定。因此,式(5)可以表达为:
-d[Imid]t/dt=kapp[Imid]t (6)
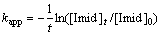 (7)
(7)
式中:kapp=kO3-Imid [O3]t+ k·OH-Imid[·OH]t,为吡虫啉在臭氧氧化过程中的准一级反应速率常数,min-1。
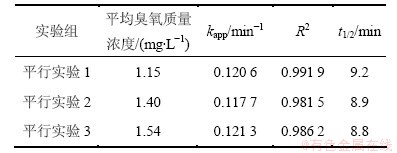
根据式(7)拟合3组平行实验中ln([Imid]t/[Imid]o)与反应时间t的变换规律,结果见表1。从表1可以看出:吡虫啉在臭氧氧化过程中的降解趋势符合准一级动力学模型,拟合相关系数均R2均大于0.980 0,且3组平行实验获得的准一级动力学常数相近,这说明在半连续反应模式下,实验结果的重现性很好。
表1 中性条件下臭氧氧化吡虫啉的准一级反应动力学常数
Table 1 Pseudo first-order rate constants for imidacloprid degradation during ozonation under neutral condition
2.2 pH和碱度对降解效果的影响
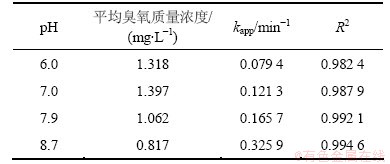
不同pH对吡虫啉降解效果的影响如图3所示,相应的准一级动力学常数kapp、平均臭氧质量浓度以及相关系数R2见表2。当吡虫啉的初始质量浓度为0.5 mg/L时,随着pH的升高,吡虫啉的降解速率也加快。当pH为6.0时,30 min内吡虫啉的降解率仅为60%,而当pH为8.7时,15 min内吡虫啉的去除率就可达到99%。随着pH从6.0增加至8.7,吡虫啉在臭氧氧化过程中的准一级降解速率常数从0.079 4 min-1增加到0.325 9 min-1,这说明碱性条件更有利于吡虫啉在臭氧氧化工艺中的降解。根据式(1)和式(2)可知:在酸性条件下,臭氧分子是主要的氧化剂,而碱性条件下,[OH-]的增加有利于羟基自由基的生成;与臭氧(氧化电位E=2.07 eV)相比,羟基自由基(氧化电位为2.85 eV[24])的氧化能力更强,氧化范围更广,其与大多数有机物反应的二级动力学常数在108~1010 (mol/L)-1·s-1范围内[22]。在碱性条件下,氧化能力更强的羟基自由基含量增加从而导致了吡虫啉降解速率的提高。
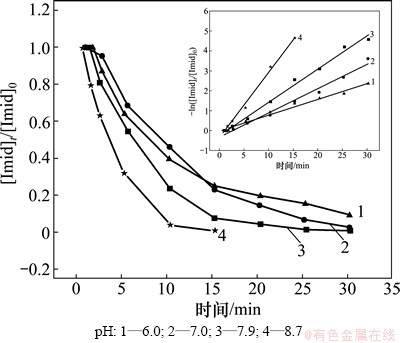
图3 不同pH条件下吡虫啉的降解效果 (内图所示为准一级动力学拟合曲线)
Fig. 3 Imidacloprid degradation by ozone at different pH values
表2 不同pH条件下吡虫啉降解的准一级速率常数
Table 2 Pseudo first-order rate constants for imidacloprid ozonation at different pH values
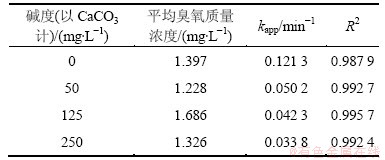
碱度对吡虫啉降解速率的影响如图4所示,相应的准一级动力学常数kapp、平均臭氧质量浓度以及相关系数R2见表3。吡虫啉的初始质量浓度为 0.5 mg/L,反应溶液pH为7.0。当碱度(以CaCO3计)从0 mg/L增加到250 mg/L时,吡虫啉在臭氧氧化过程中的准一级降解速率常数从0.121 3 min-1降低到0.033 8 min-1,这说明碱度的增加会抑制吡虫啉在臭氧氧化过程中的降解。碳酸盐和碳酸氢盐是很强的羟基自由基捕获剂,如式(8)和式(9)[22]所示。一方面,它们可以与吡虫啉竞争羟基自由基,从而减少羟基自由基对吡虫啉的氧化作用;另一方面,碳酸根和碳酸氢根与羟基自由基反应后生成的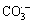 不会进一步激发生成羟基自由基的链式反应[25],从而也减少了羟基自由基的生成量。
不会进一步激发生成羟基自由基的链式反应[25],从而也减少了羟基自由基的生成量。
·OH+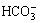 →
→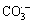 +H2O,k3=8.5×106 (mol/L)-1·s-1 (8)
+H2O,k3=8.5×106 (mol/L)-1·s-1 (8)
·OH+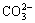 →
→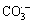 +OH-,k4=3.9×108 (mol/L)-1·s-1 (9)
+OH-,k4=3.9×108 (mol/L)-1·s-1 (9)
从pH和碱度对降解效果的影响也可以得出:羟基自由基对于吡虫啉的降解起到了非常重要的作用。因此,在实际应用中,可以通过适当增加pH、与紫外辐照联用或加入H2O2等措施增加羟基自由基的含量,从而提高对吡虫啉的降解效率。为了进一步验证这一推论,本文分别研究吡虫啉与臭氧分子和羟基自由基反应的二级动力学特性。
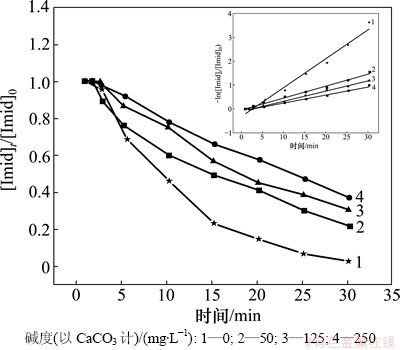
图4 不同碱度条件下吡虫啉的降解效果 (内图所示为准一级动力学拟合曲线)
Fig. 4 Imidacloprid degradation by ozone at different alkalinity concentrations
表3 不同碱度条件下吡虫啉降解的准一级速率常数
Table 3 Pseudofirst-order rate constants for imidacloprid ozonation at different alkalinity concentrations
2.3 吡虫啉与臭氧分子反应的动力学研究
在吡虫啉初始质量浓度为0.5 mg/L,pH为2.0,加入50 mmol/L叔丁醇的条件下测定吡虫啉与臭氧分子的二级反应动力学常数。由式(1)和式(2)可知:强酸性条件可以抑制羟基自由基的生成,而叔丁醇(TBA)与臭氧反应非常缓慢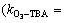 0.001 (mol/L)-1·s-1),但可以与羟基自由基迅速反应[26-27],因此,可以极大地抑制羟基自由基对吡虫啉的氧化降解作用。在这一实验过程中,可以认为臭氧分子是主要的氧化剂,因此,吡虫啉的降解速率可以表达为:
0.001 (mol/L)-1·s-1),但可以与羟基自由基迅速反应[26-27],因此,可以极大地抑制羟基自由基对吡虫啉的氧化降解作用。在这一实验过程中,可以认为臭氧分子是主要的氧化剂,因此,吡虫啉的降解速率可以表达为:
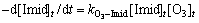 (10)
(10)
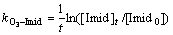 (11)
(11)
实验结果如图5所示。由图5可以看出:吡虫啉在臭氧分子的氧化作用下降解速率比较缓慢,当臭氧质量浓度分别为0.371 mg/L和0.325 mg/L时,30 min内吡虫啉的降解率仅为15.2%和12.4%。利用式(11)计算得到吡虫啉与臭氧分子反应的二级动力学常数为(10.92±0.12) (mol/L)-1·s-1。臭氧分子对有机物的氧化具有较强的选择性,对于含有供电子基团(如不饱和键、活性芳香环或无质子胺类等)的有机物,臭氧分子易于将其氧化;而含有得电子基团(如—Cl,—NO2,—SO2)的有机物则不易被臭氧分子氧化,因为这些基团的电负性较高会增加官能团上的电子密度,从而抑制臭氧分子对其的氧化作用[22]。吡虫啉分子中的咪唑烷环上含有易于被臭氧分子氧化的三级胺基团和二级胺基团,但氯代吡啶环上的—Cl和咪唑烷环上的—NO2基团会抑制臭氧分子对吡虫啉的氧化作用。由文献[22]可知,臭氧分子与有机物反应的二级动力学常数在<0.1到7×109 (mol/L)-1·s-1范围内,吡虫啉与臭氧分子反应的二级动力学常数比较低,这可能是—Cl和—NO2基团的抑制作用强于三级胺和二级胺基团的促进作用所致。Bourgin等[28]的研究表明吡虫啉在臭氧氧化初期生成的产物均含有完整的氯代吡啶环,这也证明了含—Cl基团的官能团不易被臭氧氧化这一理论。
2.4 吡虫啉与羟基自由基反应的动力学研究
在臭氧浓度为4.0×10-6 mol/L,过氧化氢浓度为2.0×10-6 mol/L条件下同时加入等浓度的吡虫啉和对氯苯甲酸以测定吡虫啉与羟基自由基反应的二级动力学常数,吡虫啉和对氯苯甲酸的初始浓度均为6.0×10-6 mol/L。选用对氯苯甲酸为竞争参照物,因为对氯苯甲酸与羟基自由基的反应非常迅速,其二级动力学常数为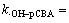 5×109 (mol/L)-1·s-1 [22],并且对氯苯甲酸和吡虫啉与臭氧分子的反应均非常缓慢(<0.15 (mol/L)-1·s-1 [27],本研究中得到的
5×109 (mol/L)-1·s-1 [22],并且对氯苯甲酸和吡虫啉与臭氧分子的反应均非常缓慢(<0.15 (mol/L)-1·s-1 [27],本研究中得到的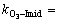 10.92 (mol/L)-1·s-1。在此实验条件下,臭氧分子引发的直接氧化过程微弱,可以忽略。为了进一步减少拟合误差,在实验过程中将臭氧质量浓度保持在较低水平(0.15 mg/L),同时加入H2O2以增加羟基自由基的产量,如式(12)和(13)所示。
10.92 (mol/L)-1·s-1。在此实验条件下,臭氧分子引发的直接氧化过程微弱,可以忽略。为了进一步减少拟合误差,在实验过程中将臭氧质量浓度保持在较低水平(0.15 mg/L),同时加入H2O2以增加羟基自由基的产量,如式(12)和(13)所示。
H2O2+H2O=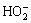 +H3O+ (12)
+H3O+ (12)
O3+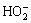 =·OH+
=·OH+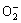 +O2 (13)
+O2 (13)
因此,在这种实验条件下,羟基自由基与吡虫啉的反应成为吡虫啉降解的主要过程。羟基自由基同时氧化吡虫啉和对氯苯甲酸的动力学方程可以表达为:
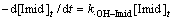 [·OH]t (14)
[·OH]t (14)
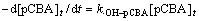 [·OH]t (15)
[·OH]t (15)
对式(14)和(15)进行积分并合并后得
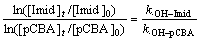 (16)
(16)
式中:[Imid]0和[Imid]t分别为吡虫啉的初始浓度和反应过程中某一时刻的浓度,mol/L;[pCBA]0和[pCBA]t分别为对氯苯甲酸的初始浓度和反应过程中某一时刻的浓度,mol/L;[·OH]t为反应溶液中羟基自由基的浓度,mol/L;k·OH-pCBA为对氯苯甲酸与羟基自由基反应的二级动力学常数(5.0×109 (mol/L)-1·s-1[20]);k·OH-Imid为吡虫啉与羟基自由基反应的二级动力学常数,(mol/L)-1·s-1。
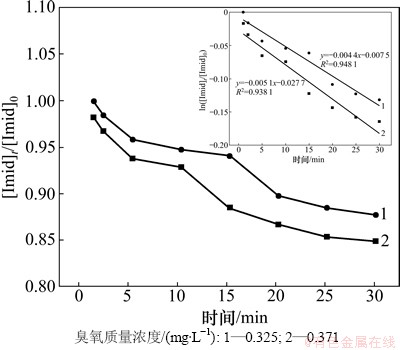
图5 吡虫啉被臭氧分子氧化的降解趋势(内图所示为吡虫啉与臭氧反应的二级动力学拟合曲线)
Fig. 5 Degradation of imidacloprid by molecular ozone oxidation
根据上述原理,本实验研究了不同pH条件下吡虫啉与羟基自由基反应的动力学特性,实验结果见图6,相应的二级速率常数和相关系数R2见表4。
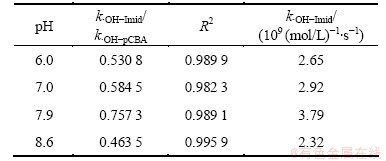
从表4可以看出:当pH为7.0时,吡虫啉与羟基自由基反应的二级动力学常数k·OH-Imid为2.92×109 (mol/L)-1·s-1,这比Zaror等[5]在光催化-Fenton氧化吡虫啉的过程中得到的结果(k·OH-Imid=4.3×109 (mol/L)-1·s-1,在pH=2.8,298 K条件下)略低,这一细微差异可能是实验条件不同导致的。这说明吡虫啉与羟基自由基的反应很迅速,这是因为吡虫啉分子中有易于被羟基自由基氧化的结构如咪唑烷环上的胺基基团和=N—NO2基团。同时,这一结果也说明羟基自由基含量的变化会对吡虫啉的降解速率造成很大的影响,因此,在实际应用中,可以通过增加羟基自由基含量来提高吡虫啉的降解效率。当pH从6.0增加到8.6时,k·OH-Imid在2.65×109~3.79×109 (mol/L)-1·s-1范围内波动,pH对羟基自由基氧化吡虫啉的速率影响不大,这表明羟基自由基氧化吡虫啉的反应位点受pH影响较小[20, 24, 29]。这一结论与Malato等[10]的研究结果一致,他们发现吡虫啉在被·OH氧化时生成的产物主要是通过攻击咪唑烷环上的三级胺C—N键或脱—NO2基等途径生成的。这些反应位点不会在碱性条件下发生去质子反应,因此,反应速率受pH影响不大。
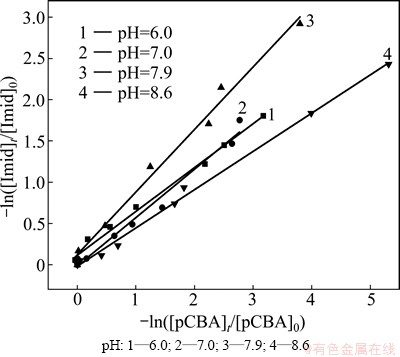
图6 不同pH条件下·OH 同时氧化对氯苯甲酸和吡虫啉的-ln([pCBA]t/[pCBA]0)与-ln([pCBA]t/[pCBA]0)的关系曲线
Fig. 6 -ln([Imid]t/[Imid]0) versus -ln([Imid]t/[Imid]0) during simultaneous oxidation of imidacloprid and pCBA by·OH at different pH values
表4 不同pH条件下吡虫啉与羟基自由基反应的二级动力学常数
Table 4 Second order rate constants of reactions between imidacloprid and hydroxyl radicals at different pH values
3 结论
1) 臭氧可以有效降解水中的吡虫啉,降解效果受溶液pH、碱度影响比较明显;当臭氧平均质量浓度为1.15 mg/L时,随着pH从6.0增加到8.6,吡虫啉降解的准一级动力学常数由0.079 min-1增至0.326 min-1;当pH为7.0,臭氧平均质量浓度为1.41 mg/L时,随着碱度(以CaCO3计)由0 mg/L增至250 mg/L时,吡虫啉降解的准一级动力学常数由0.121 min-1降至0.034 min-1。
2) 吡虫啉与臭氧分子和羟基自由基的反应符合二级动力学模型,在pH为2.0,加入50 mmol/L叔丁醇条件下,臭氧分子与吡虫啉反应的二级动力学常数为(10.92±0.12) (mol/L)-1·s-1;当pH为7.0时,吡虫啉与羟基自由基反应的二级动力学常数为2.92×109 (mol/L)-1·s-1;当pH在6.0~8.6范围内变化时,此二级反应动力学常数在2.65×109~3.79×109 (mol/L)-1·s-1范围内波动。
参考文献:
[1] 唐建军, 陈欣, 张传进, 等. 超高效杀虫剂吡虫啉的特性及其应用[J]. 中国农学通报, 1999(1): 38-40.
TANG Jianjun, CHEN Xin, ZHANG Chuanjin, et al. Advances of imidacloprid research and its applicationin in crop pest control[J]. Chinese Agricultural Science Bulletin, 1999(1): 38-40.
[2] Dryden M W, Denenberg T M, Bunch S. Control of fleas on naturally infested dogs and cats and in private residences with topical spot applications of fipronil or imidacloprid[J]. Veterinary Parasitology, 2000, 93(1): 69-75.
[3] 马国欣, 王成龙, 范多旺, 等. 红外光谱法测定农药中吡虫啉含量[J]. 光谱学与光谱分析, 2006, 26(3): 434-437.
MA Guoxin, WANG Chenglong, FAN Duowang, et al. Quantitative determination of imidacloprid by infrared absorption spectrometry[J]. Spetroscopy and Spectral Analysis, 2006, 26(3): 434-437.
[4] 徐尚成. 烟碱类杀虫剂的研究与开发进展[J]. 精细与专用化学品, 2001, 9(5): 3-5.
XU Shangcheng. Development of neonicotinoid insecticide[J]. Fine and Specialty Chemicals, 2001, 9(5): 3-5.
[5] Zaror C, Segura C, Mansilla H, et al. Kinetic study of Imidacloprid removal by advanced oxidation based on photo-Fenton process[J]. Environmental Technology, 2010, 31(13): 1411-1416.
[6] Wamhoff H, Schneider V. Photodegradation of imidacloprid[J]. Journal of Agricultural and Food Chemistry, 1999, 47(4): 1730-1734.
[7] Kitsiou V, Filippidis N, Mantzavinos D, et al. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions[J]. Applied Catalysis B (Environmental), 2009, 86(1/2): 27-35.
[8] Thuyet D Q, Watanabe H, Yamazaki K, et al. Photodegradation of imidacloprid and fipronil in rice-paddy water[J]. Bulletin of Environmental Contamination and Toxicology, 2011, 86(5): 548-553.
[9] Anhalt J C, Moorman T B, Koskinen W C. Biodegradation of imidacloprid by an isolated soil microorganism[J]. Journal of Environmental Science and Health Part B—Pesticides Food Contaminants and Agricultural Wastes, 2007, 42(5): 509-514.
[10] Malato S, Caceres J, Aguera A, et al. Degradation of imidacloprid in water by photo-fenton and TiO2 photocatalysis at a solar pilot plant: A comparative study[J]. Environmental Science & Technology, 2001, 35(21): 4359-4366.
[11] Segura C, Zaror C, Mansilla H D, et al. Imidacloprid oxidation by photo-Fenton reaction[J]. Journal of Hazardous Materials, 2008, 150(3): 679-686.
[12] Liu W P, Zheng W, Ma Y, et al. Sorption and degradation of imidacloprid in soil and water[J]. Journal of Environmental Science and Health Part B—Pesticides Food Contaminants and Agricultural Wastes, 2006, 41(5): 623-634.
[13] Zahoor M, Mahramanlioglu M. Adsorption of imidacloprid on powdered activated carbon and magnetic activated carbon[J]. Chemical and Biochemical Engineering Quarterly, 2011, 25(1): 55-63.
[14] Benner J, Ternes T A. Ozonation of metoprolol: Elucidation of oxidation pathways and major oxidation products[J]. Environmental Science & Technology, 2009, 43(14): 5472-5480.
[15] Rahman M F, Yanful E K, Jasim S Y, et al. Advanced oxidation treatment of drinking water: Part I. Occurrence and removal of pharmaceuticals and endocrine-disrupting compounds from lake huron water[J]. Ozone-Science & Engineering, 2010, 32(4): 217-229.
[16] Ormad M P, Miguel N, Lanao M, et al. Effect of application of ozone and ozone combined with hydrogen peroxide and titanium dioxide in the removal of pesticides from water[J]. Ozone-Science & Engineering, 2010, 32(1): 25-32.
[17] Sharma V K, Graham N J D. Oxidation of amino acids, peptides and proteins by ozone: A review[J]. Ozone-Science & Engineering, 2010, 32(2): 81-90.
[18] Coelho A D, Sans C, Esplugas S, et al. Ozonation of NSAID: A biodegradability and toxicity study[J]. Ozone-Science & Engineering, 2010, 32(2): 91-98.
[19] Broseus R, Vincent S, Aboulfadl K, et al. Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment[J]. Water Research, 2009, 43(18): 4707-4717.
[20] Chelme-Ayala P, El-Din M G, Smith D W, et al. Oxidation kinetics of two pesticides in natural waters by ozonation and ozone combined with hydrogen peroxide[J]. Water Research, 2011, 45(8): 2517-2526.
[21] 王业耀, 王占生. 靛红钾法测定水中的臭氧浓度[J]. 中国给水排水, 2003, 19(4): 95-97.
WANG Yeyao, WANG Zhansheng. Indigo determination of ozone in water[J]. China Water and Wastewater, 2003, 19(4): 95-97.
[22] von Gunten U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation[J]. Water Research, 2003, 37(7): 1443-1467.
[23] Elovitz M S, von Gunten U. Hydroxyl radical ozone ratios during ozonation processes. I: The R-ct concept[J]. Ozone-Science & Engineering, 1999, 21(3): 239-260.
[24] Sui M H, Xing S C, Zhu C Y, et al. Kinetics of ozonation of typical sulfonamides in water[J]. Biomedical and Environmental Sciences, 2011, 24(3): 255-260.
[25] Staehelin J, Hoigne J. Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions[J]. Environmental Science & Technology, 1985, 19(12): 1206-1213.
[26] Hoigné J, Bader H. The role of hydroxyl radical reactions in ozonation processes in aqueous solutions[J]. Water Research, 1976, 10(5): 377-386.
[27] Yao C C D, Haag W R. Rate constants for direct reactions of ozone with several drinking water contaminants[J]. Water Research, 1991, 25(7): 761-773.
[28] Bourgin M, Violleau F, Debrauwer L, et al. Ozonation of imidacloprid in aqueous solutions: Reaction monitoring and identification of degradation products[J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 60-68.
[29] Vel Leitner N K, Roshani B. Kinetic of benzotriazole oxidation by ozone and hydroxyl radical[J]. Water Research, 2010, 44(6): 2058-2066.
(编辑 杨幼平)
收稿日期:2013-11-25;修回日期:2014-01-09
基金项目(Foundation item):国家科技重大专项(2012ZX07403-001, 2012ZX07403-002, 2008ZX07421-002);国家自然基金资助项目(51178321);高等学校博士点基金资助项目(20120072110050)(Projects (2012ZX07403-001, 2012ZX07403-002, 2008ZX07421-002) supported by National Major Project of Science & Technology Ministry of China; Project (51178321) supported by National Natural Science Foundation of China; Project (20120072110050) supported by Research Fund for Doctoral Program of Higher Education of China)
通信作者:高乃云(1950-),女,陕西府谷人,博士,教授,博士生导师,从事饮用水处理技术和建筑给水排水技术研究;电话:13816869935;E-mail: gaonaiyun1@126.com