


Trans. Nonferrous Met. Soc. China 22(2012) 2289-2294
Preparation of α-Bi2O3 from bismuth powders through low-temperature oxidation
XIA Ji-yong1, TANG Mo-tang1, CHEN Cui1, JIN Sheng-ming2, CHEN Yong-ming1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 23 September 2011; accepted 5 January 2012
Abstract: α-Bi2O3 powders were prepared from nanometer Bi powders through low-temperature oxidation at less than 873.15 K. XRD, SEM, TEM and HRTEM were used to characterize the structure and morphology of Bi powders and Bi2O3 particles. Kinetic studies on the bismuth oxidation at low-temperatures were carried out by TGA method. The results show that bismuth beads should be reunited and oxidized to become irregular Bi2O3 powders. The bismuth oxidation follows shrinking core model, and its controlling mechanism varies at different reaction time. Within 0-10 min, the kinetics is controlled by chemical reaction, after that it is controlled by O2 diffusion in the solid α-Bi2O3 layer. The apparent activation energy is determined as 55.19 kJ/mol in liquid-phase oxidation.
Key words: bismuth powder; low-temperature oxidation; α-Bi2O3; oxidation kinetics
1 Introduction
Bi2O3 has been used widely in the fields of catalysis [1,2], functional ceramics [3-7], special glass and optical materials [8-11], medicine synthesis [12], energy materials [13,14] and superconductor materials [15,16] owing to its special properties of wide band gap, dielectric permittivity, high refractive index, photoluminescence and photoconductivity. Many methods such as high-temperature oxidation of bismuth metal [17-19], pyrolysis of bismuth compound [20,21], hydro-chemical method [22-25] and solid state reaction [26] have been reported to prepare Bi2O3 powders. High-temperature oxidation of bismuth metal is also dividable into vapor-oxidation and spray-oxidation. Beta- and alpha- phase Bi2O3 nanoparticles could be formed through high-temperature vapor-oxidation of Bi metal at 1278 K [17]. It was difficult to oxidize bismuth completely to form Bi2O3 through spray-oxidation due to short retention time in air and kinetics limit at 1073 K [19]. Bi2O3 particles with hollow and well-defined morphology could be synthesized through spray-pyrolysis process with change of pyrolysis temperature using Bi(NO3)3·5H2O as raw materials. However, some above mentioned methods have disadvantages like higher energy consumption, complicated equipments and processing method, and the others produce toxic waste like NxOy gas.
Preparation of Bi2O3 at less than 873 K was investigated using metallic bismuth as raw materials. The reaction temperature can be controlled lower than 873 K in low-temperature oxidation process. Consequently, energy consumption and equipment cost obviously decrease. Moreover, low-temperature oxidation method could avoid producing air-borne emissions in spray-pyrolysis and reduce the waste water discharge in hydro-chemical method. The present work aims at investigating the kinetics of bismuth oxidation at a low temperature and characterizing the structure of Bi2O3 powders obtained from this method. The studies are a useful guideline on preparing Bi2O3 powders at less than 873.15 K, understanding the oxidation mechanism of bismuth and increasing the yield of Bi2O3 particles through low-temperature oxidation process.
2 Experimental
The bismuth powders with particle size of 20 nm were prepared through fusion spraying method. Oxidation of bismuth at a low temperature was conducted by a Q600 differential thermal analyzer (TA, USA). Bismuth powders were put into the thermal analyzer and treated at a predetermined temperature for 30 min in N2 flow with a heating rate of 3 ℃/min, and then, switched from N2 flow to air flow. The mass change of bismuth powder was recorded and the oxidation ratio of bismuth powders was calculated according to equation (1).
α=(2×208.9804×?m)/(3×15.9994×m)=8.7075×?m/m (1)
where α denotes the fraction of oxidized bismuth, m is the initial mass of bismuth powders, and ?m represents the increased mass of the bismuth powders after oxidation reaction at different temperatures. The chemical compositions of Bi powders and as-synthesized Bi2O3 are listed in Table 1, which were analyzed by ICP-AES.
Table 1 Chemical compositions of bismuth powders and Bi2O3

The X-ray diffraction (XRD) of the sample was recorded on a D/max 2550 (Rigaku, Japan) with Cu Kα radiation (λ=0.1548 nm) from 10°~80°(2θ) at a scanning step of 0.25 (°)/s. The morphologies of the samples were characterized by a high-resolution transmission electron microscope (Tecnai G2 20, FEI, USA). Particle size distribution was detected by Mastersizer 2000 (Malvern, England).
3 Results and discussion
3.1 Effect of oxidation temperature on formation of Bi2O3
The bismuth-oxidation reaction can be presented as:
2Bi(s)+1.5O2(g)=Bi2O3(s) (298-544 K), (1)
?GΘ=-574887+99.71T
2Bi(l)+1.5O2(g)=Bi2O3(s) (544-1090 K), (2)
?GΘ=-589730+292.4T
2Bi(l)+1.5O2(g)=Bi2O3(l) (1090-1600 K), (3)
?GΘ=-535418+243.7T
According to equations (1), (2), and (3), the Gibbs free energy is minus in the temperature range of 200-1600 K, which means the bismuth oxidation reaction is thermodynamically spontaneous.
Figure 1 shows the effect of oxidation time on yield of bismuth oxide at different temperatures under the conditions of particle size of 20 nm and air flow of 35 mL/min. The results demonstrate that the oxidation rate of bismuth powder increases with the increase of temperature. At 450 ℃, the oxidation rate of bismuth is only 83.17% after 187 min. At 600 ℃, however, the bismuth is completely transferred f electrodeposited to Bi2O3 even through the reaction time is only 15 min. The possible reasons for these phenomena may be as follows: the O2 diffusion coefficient in the gas film and solid Bi2O3 layer increases with the increase of temperature. Consequently, the oxygen molecules can rapidly pass through the gas film and solid Bi2O3 layer and transfer to the surface of un-reacted bismuth core. Sufficient oxygen molecules are supplied for the oxidation of bismuth powders.
The results propose that oxidation process of bismuth is controlled by gaseous and liquid oxidation, and gaseous oxidation contributes to improving oxidation rate. The relationship between vapor pressure of melting Bi and temperature is as follows [27]:
lg p=-10051/T+8.462 (4)
where p denotes the vapor pressure of melting Bi, and T is the thermodynamic temperature.
According to Eq. (4), the vapor pressures of Bi are 90.18 Pa at 873.15 K, 18.03 Pa at 823.15 K, 2.92 Pa at 773.15 K, 0.37 Pa at 723.15 K, respectively. Higher vapor pressure can destroy the film outside of the bismuth bead and benefit diffusion of O2 molecules from atmosphere to surface of bismuth bead.

Fig. 1 Effect of oxidation time on yield of bismuth oxide at different temperatures
3.2 Particle size distribution and crystal structure of Bi2O3
The XRD pattern of as-prepared Bi2O3 is shown in Fig. 2. Three characteristic peaks are indexed as (120), (200) and (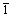 21), indicating that monoclinic Bi2O3 (PDF# 65—2366, P21/c(14)) has come into being. The XRD results demonstrate that there are no impurities existing in powders.
21), indicating that monoclinic Bi2O3 (PDF# 65—2366, P21/c(14)) has come into being. The XRD results demonstrate that there are no impurities existing in powders.
Laser particle analysis shows that particle size distribution ranging from 10 to 70 μm is the dominating size characteristic, and a few particle sizes are less than 100 nm (see Fig. 3).

Fig. 2 XRD pattern of as-synthesized Bi2O3
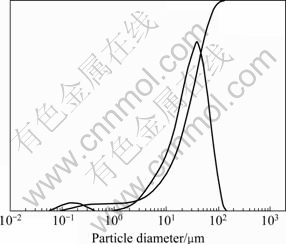
Fig. 3 Laser particle size distribution analysis of as-synthesized Bi2O3
To understand the structure of as-synthesized α-Bi2O3 and oxidation mechanism of Bi particles better, the TEM images of Bi particles, SEM and HRTEM images of α-Bi2O3 powders are shown in Fig. 4.
Figure 4(a) shows that raw materials of Bi powders are nano-particles with regular shape. Melting bismuth will become spherical at a high temperature due to surface tension. The shape of α-Bi2O3 would be spherical if oxidation process in-situ could occur. However, the SEM image of α-Bi2O3 powders shows that the shape is irregular (see Fig. 4(b)). Particles reunite each other, and some small narrow particles cohere to large size α-Bi2O3 powders. Results propose further that oxidation of bismuth involves two types of processes namely vapor-phase and liquid-phase. The latter dominates the whole process while the former controls the shape of cohered α-Bi2O3 powders.
Figure 4(c) shows a HRTEM image with inset of FFT from a α-Bi2O3 particle. This image clearly displays that the lattice fringes of as-synthesized α-Bi2O3 have no defect appearance. The observed HRTEM image shows a lattice spacing of 0.268 nm and 0.325 nm, corresponding to that of (200) plane and (120) of α-Bi2O3. The FFT image of HRTEM shows a set of diffraction pots with typical monoclinic structure. The result further confirms that the powders are α-Bi2O3 crystalline.

Fig. 4 TEM image of Bi powders (a), SEM image (b) and HRTEM image (c) of as-synthesized α-Bi2O3 (Inset of (c) is FFT image of HRTEM)
3.3 Kinetics of bismuth oxidation at low temperature
The process was dominated by liquid-phase oxidation. The mechanism of bismuth oxidation is demonstrated in Fig. 5, and the formation of α-Bi2O3 at a low temperature can be divided into four steps.
1) Solid bismuth powders fuse to form bismuth beads, and bismuth beads reunite each other. Oxygen molecules in air flow diffuse into gas film which covers the surface of bismuth beads. After passing through the gas film, the oxygen molecules absorb on the surface of bismuth beads.

Fig. 5 Schematic representation of oxidation mechanism of bismuth powders
2) Oxygen molecules absorbing on the bismuth beads crack into O atoms, and then react with bismuth to form Bi2O3, which covers the un-reacted bismuth core to form solid-state layer.
3) Oxygen molecules continue diffusing through the gas film and solid Bi2O3 layer in turn, and react with liquid bismuth beads. Consequently, the volume of un-reacted bismuth core shrinks continuously.
4) Oxidation reaction of bismuth powders is finished, and the bismuth beads convert into solid Bi2O3 completely.
Since the whole process mentioned above is similar to the chemical reaction between compact solid particle and gas, the un-reacted shrinking core model can be used to describe the formation of Bi2O3. The reaction controlling steps during formation of Bi2O3 at a low temperature are as follows.
1) If the reaction rate is controlled by O2 diffusion in the gas film, the kinetic equation is α=kt.
2) If the reaction rate is controlled by O2 diffusion in the Bi2O3 layer, the kinetic equation can be presented as 1-3(1-α)2/3+2(1-α)=kt.
3) If the reaction rate is controlled by chemical reaction, the kinetic equation is 1-(1-α)1/3=kt.
4) If the reaction rate is the mixture controlling of chemical reaction and solid phase diffusion, the kinetic equation can be presented as t=[1-3(1-α)2/3+2(1-α)]/k2+ [1-(1-α)1/3]/k3.
The linear fitting of oxidation curve of bismuth powder at different temperatures is presented in Fig. 6 and Fig. 7. In the temperature range of 450-600 ℃, a linear relationship between 1-(1-α)1/3 and reaction time (t) is obtained within 0-10 min (see Fig. 6). After 10 min, a plot of 1-3(1-α)2/3+2(1-α) versus reaction time (t) shows another perfect linear relationship (see Fig. 7). It indicates that the bismuth oxidation follows shrinking core model, and its controlling mechanism is variable at different reaction time. Within 0-10 min, the kinetics is controlled by the chemical reaction, and then with the oxidation reaction proceeding, it is controlled by O2 diffusion in the solid Bi2O3 layer.
However, the oxidation mechanism does not correspond to the above kinetics equation when the oxidation process occurs at 823.15 K and 873.15 K after 10 min. These phenomena confirm that more complex kinetics mechanism exists in oxidation reaction of Bi powders at a higher temperature. The oxidation reaction rate constants of bismuth powders at different temperatures are listed in Table 2. It shows that the oxidation rate constant increases sharply with the increase of temperature during the chemical reaction controlling period. As shown in Fig. 8, a plot of the ln k versus T-1 displays a perfect linear relationship, and the apparent activation energy is determined to be 55.19 kJ/mol according to Arrhenius equation.
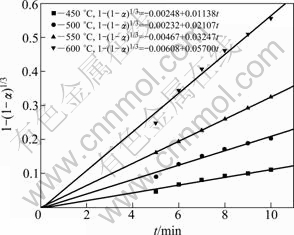
Fig. 6 Linear relationship between 1-(1-α)1/3 and t within 0-10 min at different temperatures

Fig. 7 Linear relationship between 1-3(1-α)2/3+2(1-α) and t at different temperatures after 10 min
Table 2 Oxidation reaction rate constants of bismuth powders at different temperatures


Fig. 8 Linear relationship between lnk and T-1
4 Conclusions
1) α-Bi2O3 powders were prepared from nano Bi powders through low-temperature oxidation at less than 873.15 K. The conversion of Bi is 100% after oxidation at 823.15 K for 40 min. Microstructural results of Bi2O3 powders show that bismuth beads should be reunited and oxidized to become irregular Bi2O3 powders. Oxidation of bismuth involves two types of processes, namely vapor-phase and liquid-phase. The latter dominates the whole process while the former controls the shape of cohered α-Bi2O3 powders.
2) Kinetic experimental results indicate that the bismuth oxidation follows the shrinking core model, and its controlling mechanism is variable at different reaction time. Within 0-10 min, the kinetics is controlled by chemical reaction, and then with the oxidation reaction proceeding, it is controlled by O2 diffusion in the solid Bi2O3 layer. The oxidation rate constant increases sharply with the increase of temperature during chemical reaction controlling period, and the apparent activation energy is determined to be 55.19 kJ/mol.
3) The low-temperature oxidation process can prepare Bi2O3. The contents of Bi2O3 and Ca, Fe, Si and Sb and other impurity elements are up to industrial standards for electronic-grade bismuth oxide.
References
[1] JIN Sheng-ming, TANG Mo-tang, YANG Wei-jun. Preparation of catalyst for ammoxidation of propylene in chlorination salts systems (Ⅱ)—Catalytic activity rating of Bi containing catalyst [J]. Journal of Central South University of Technology: Natural Science, 2001, 32(4): 247-250. (in Chinese)
[2] YANG Han-pei, FAN Yi-ning, LIN Ming, XU Bo-lian, CHEN Yi. Structure and catalytic properties of Bi-Mo composite oxide catalyst for selective oxidation of propane [J]. Chinese Journal of Catalysis, 2002, 60(6): 1006-1010. (in Chinese)
[3] LAZAREVI? Z, STOJANOVI? B D, VARELA J A. An approach to analyzing synthesis, structure and properties of bismuth titanate ceramics [J]. Science of Sintering, 2005, 37(3): 199-216.
[4] PAVLOVIC NIKOLINA, SRDIC VLADIMIR V. Synthesis and structural characterization of Ce-doped bismuth titanate [J]. Materials Research Bulletin, 2009, 44(4): 860-864.
[5] LAURENT K, WANG G Y, TUSSEAU-NENEZ S, LEPRINCE-WANG Y. Structure and conductivity studies of electrodeposited δ-Bi2O3[J]. Solid State Ionics: Diffusion and Reactions, 2008, 178(33): 1735-1739.
[6] SHAO Zong-ping, XIONG Guo-xing, YANG Wei-shen. Progress in bismuth-contained mixed conducting oxide membranes [J]. Journal of Inorganic Materials, 2001, 16(1): 23-31.
[7] SUNARSO J, LIU S, LIN Y S, da COSTA J C D. Oxygen permeation performance of BaBiO3-δ ceramic membranes [J]. Journal of Membrane Science, 2009, 344(1-2): 281-287.
[8] CHEN Dan-ping, JIANG Xiong-wei, ZHU Cong-shan. Study on the structure of Bi2O3-Li2O glass [J]. Journal of Inorganic Materials, 2002, 17(2): 202-209.
[9] YANG Jian-hu, DAI Shi-xun, WEN Lei, LIU Zhu-ping, HU Li-li, JIANG Zhong-hong. Spectroscopic properties and thermal stability of a new erbium-doped bismuth-based glass [J]. Acta Physica Sinica, 2003, 52(3): 508-514.
[10] LEONTIE L, CARAMAN M, DELIBAS M, RUSU G I. Optical properties of bismuth trioxide thin films [J]. Materials Research Bulletin, 2001, 36(9): 1629-1637.
[11] HASEGAWA T, NAGASHIMA T, SUGIMOTO N. Determination of nonlinear coefficient and group-velocity-dispersion of bismuth-based high nonlinear optical fiber by four-wave-mixing [J]. Optics Communications, 2008, 281(4): 782-787.
[12] YANG Nan, SUN Hong-zhe. Biocoordination chemistry of bismuth: Recent advances [J]. Coordination Chemistry Reviews, 2007, 25(17-20): 2354-2366
[13] JIANG Zhi-yi, ZHANG Lei, CAI Li-li, XIA Chang-rong. Bismuth oxide-coated (La,Sr)MnO3 cathodes for intermediate temperature solid oxide fuel cells with yttria-stabilized zirconia electrolytes [J]. Electrochimica Acta, 2009, 54(11): 3059-3065.
[14] SMIRNOVA A, SARAT S, SAMMES N. Bismuth oxide doped scandia-stabilized zirconia electrolyte for the intermediate temperature solid oxide fuel cells [J]. Journal of Power Sources, 2006, 160(2): 892-896.
[15] LIN Yu-bao, LIN Liang-zhen, XIAO Li-ye. Development of bismuth-base high temperature superconducting DC cable [J]. Physics, 2001, 30(7): 389-391.
[16] MAKAROVA M V, KAZIN P E, TRETYAKOV YU D, JANSEN M, REISSNER M, STEINER W. Zr, Hf, Mo and W-containing oxide phases as pinning additives in Bi-2212 superconductor [J]. Physica C, 2005, 419(1): 61-69.
[17] HU Han-xiang, QIU Ke-qiang, XU Guo-fu. Preparation of nanometer δ- and β-bismuth trioxide by vacuum vapor-phase oxidation [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(1): 173-177.
[18] MARTIROSYAN K S, WANG L, VICENT A, LUSS D. Synthesis and performance of bismuth trioxide nanoparticles for high energy gas generator use[J]. Nanotechnology, 2009, 20(40): 405-412.
[19] KR?GER J, WINKLER P, LU¨DERITZ E, L?CK M, WOLF H U. Bismuth, bismuth alloys, and bismuth compounds, Ullmann’s encyclopedia of industrial chemistry [M]. Weinheim, Germany: Wiley–VCH Verlag GmbH, 2000.
[20] M?DIER L, PRATSINIS S E. Bismuth oxide nano-particles by flame spray pyrolysis [J]. Journal of American Ceramic Society, 2002, 85(7): 1713-1718.
[21] JUNGK H, FELDMANN C F. Polymediated synthesis of submicrometer Bi2O3 particles [J]. Journal of Material Science, 2001, 36(1): 297-299.
[22] HE Wei-ming, ZHEN Qiang, PAN Qing-yi, LIU Jian-qiang. Sduty on preparation of ultra-fine bismuth oxide powder by reversing-titration method [J]. Journal of Functional Materials, 2003, 34(6): 702-706. (in Chinese)
[23] LI Wei. Preparation of monodisperse nanometer Bi2O3 powder[J]. Journal of Central South University: Science and Technology, 2005, 36(2): 175-178. (in Chinese)
[24] ZHENG Bo, REN Zhi-gang, TONG Jian-ying, GU Jian-sheng. Preparation of ultrafine bismuth oxide by electrochemical method [J]. Chemical Research and Application, 2004, 16(3): 411-412.
[25] ZHENG Bo, PANG Ai-hong, GU Jian-sheng. Synthesis of ultrafine γ-Bi2O3 powder at low temperature [J]. Chemical Journal of Chinese Universities, 2005, 26(4): 628-630.
[26] HONG Wei-liang, ZHAO Feng-qi, LIU Jian-hong, TIAN De-yu. Synthesis of nanometer PbO, Bi2O3 and their effect on burning properties of solid propellants [J]. Chinese Journal of Explosives & Propellants, 2001, 24(3): 7-9. (in Chinese)
[27] ENDEBROCK R W, ENGLE P M. Separation of polonium from bismuth by distillation[R]. AECD-4146, 1953-08-01.
铋粉低温氧化制备α-Bi2O3
夏纪勇1,唐谟堂1,陈 萃1,金胜明2,陈永明1
1. 中南大学 冶金科学与工程学院,长沙 410083;
2. 中南大学 资源加工与生物工程学院,长沙 410083
摘 要:纳米金属铋粉在低于873.15 K的温度下被氧化而制备成α-Bi2O3粉体,采用XRD、SEM、TEM和HRTEM 等技术表征α-Bi2O3 粉体的晶体结构和形貌,通过TGA技术研究铋粉的低温氧化动力学行为。结果表明,纳米铋粉在较低的温度下熔融成铋珠,铋珠结合长大并氧化生成不规则的Bi2O3 粉体,铋珠氧化机理符合核收缩模型;动力学控制步骤随着氧化时间的变化而变化,在0~10 min内,铋珠氧化动力学表现为化学反应控制,然后转化为O2内扩散控制,低温氧化表观反应活化能为55.19 kJ/mol。
关键词:铋粉;低温氧化;α-Bi2O3;氧化动力学
(Edited by LI Xiang-qun)
Foundation item: Project (2006BAB02B05-04- 01/02) supported by the National Key Technologies R&D Program of China
Corresponding author: JIN Sheng-ming; Tel: +86-731-88877204; Fax: +86-731-88710804; E-mail: shmjin@csu.edu.cn
DOI: 10.1016/S1003-6326(11)61462-3