Electrochemical deposition of aluminum on W electrode fromAlCl3-NaCl melts
来源期刊:中国有色金属学报(英文版)2010年第1期
论文作者:阚洪敏 王兆文 王晓阳 张宁
文章页码:158 - 164
Key words:aluminum; electrodeposition; intermetallic compounds
Abstract: Electrochemical deposition of aluminum on W electrode from AlCl3-NaCl melts was studied by cyclic voltammetry and chronopotentiometry. The results show that Al (Ⅲ) is reduced in two consecutive steps, i.e., 4Al2Cl7-+3e-→Al+7AlCl4- and then AlCl4-+3e-→Al+4Cl-. The electrochemical reaction of 4Al2Cl7-+3e-→Al+7AlCl4- is reversible. Certain nucleation overpotential is required during the deposition of aluminum on W electrode. Chronopotentiometry analysis also shows that Al (Ⅲ) is reduced in two consecutive steps under certain current density, which is in reasonable agreement with cyclic voltammograms. By using constant current deposition, the electrodeposits on Al substrate obtained at between 50 and 100 mA/cm2 are quite dense and well adherent to the Al substrate. The electrochemical deposition of aluminum on Cu substrate in AlCl3-NaCl melts indicates that the intermetallic compounds are formed. The intermetallic compounds are AlCu and Al2Cu.
基金信息:the National Natural Science Foundation of China
the National Basic Research Program of China

KAN Hong-min(阚洪敏)1, WANG Zhao-wen(王兆文)2, WANG Xiao-yang(王晓阳)1, ZHANG Ning(张 宁)1
1. Key Laboratory of Advanced Materials Preparation Technology of Liaoning Province,
Shenyang University, Shenyang 110044, China;
2. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 1 December 2008; accepted 19 May 2009
Abstract: Electrochemical deposition of aluminum on W electrode from AlCl3-NaCl melts was studied by cyclic voltammetry and chronopotentiometry. The results show that Al (Ⅲ) is reduced in two consecutive steps, i.e., 4Al2Cl7-+3e-→Al+7AlCl4- and then AlCl4-+3e-→Al+4Cl-. The electrochemical reaction of 4Al2Cl7-+3e-→Al+7AlCl4- is reversible. Certain nucleation overpotential is required during the deposition of aluminum on W electrode. Chronopotentiometry analysis also shows that Al (Ⅲ) is reduced in two consecutive steps under certain current density, which is in reasonable agreement with cyclic voltammograms. By using constant current deposition, the electrodeposits on Al substrate obtained at between 50 and 100 mA/cm2 are quite dense and well adherent to the Al substrate. The electrochemical deposition of aluminum on Cu substrate in AlCl3-NaCl melts indicates that the intermetallic compounds are formed. The intermetallic compounds are AlCu and Al2Cu.
Key words: aluminum; electrodeposition; intermetallic compounds
1 Introduction
Conventional Hall–Héroult electrolysis process for the aluminium production is usually operated at very high temperature (950-1 000 ℃, nowadays 930-960 ℃) and unavoidably shows high energy consumption, complicated operation and pollutant emission. Many researchers turned their interest in the methods at or near ambient temperature to reduce pollutant emission and energy consumption. Aluminium electrodeposition in organic solutions, such as aromatic hydrocarbons and ether, was investigated for potential application in aluminium refining and recycling[1]. But, the electrolyte properties (e.g., low electrochemical potential windows and low electrical conductivity) limited their applications. Over the last decade, the developments in ionic liquids[2-3] resulted in another potential approach for aluminium extraction and refining that offers potential advantages, such as, low energy consumption, low pollutant emission and low operating cost[4]. Several ionic liquids have been reported in literature as the electrolytes for the electrodeposition of aluminium[5-9]. The baths tend to be expensive, extremely water sensitive and difficult to be purified, limiting their application. The AlCl3-[EMIm]Cl ionic liquids have large composition range at room temperature and possess a number of attractive features. It is difficult to prepare due to the highly exothermic reaction occurring between AlCl3 and [EMIm]Cl components[8-9].
The AlCl3-NaCl melts possess a number of attractive features, such as low vapor pressure, relatively high electrical conductivity and wide electrochemical potential window. The electrical conductivity of an equimolar mixture of AlCl3 and NaCl melt is 0.44 S/cm at 175 ℃[10]. These favorable properties render the AlCl3-NaCl melts as potential electrolytes for the electrolytic extraction and recycling of aluminium. The AlCl3-NaCl melts have been employed as electrolytes for electroplating of metals and rechargeable batteries. Several investigators have examined the electrodeposition of pure aluminum from the AlCl3- alkali metal chloride systems[10-15]. The electrochemical behavior of aluminum in chloroaluminate melts has been studied using different primary aluminum electrodes[12-16]. In neutral melts of equimolar mixture of AlCl3 and NaCl, the dominant ionic species are Na+ and AlCl4-. In basic melts, the relative concentrations deviate from equimolar. Cl- is introduced because of excess NaCl. In acidic melts, aluminum complexes, such as Al2Cl7-, are present with excess AlCl3. The chemical equilibria operative in AlCl3-NaCl melts in a wide range of AlCl3 concentrations above the equimolar point are well known. This melt is often considered an acid-base system where the acid (Al2Cl7-) is defined as a chloride ion acceptor and the base (AlCl4-) is defined as a chloride ion donor, 2AlCl4-![]() Al2Cl7-+Cl-.
Al2Cl7-+Cl-.
The purpose of the present work is to determine the mechanism of the electrodeposition of aluminum from AlCl3-NaCl melts on W substrate. The methods of cyclic voltammetry and chronopotentiometry are employed. It is necessary to reach a better understanding of electrochemical reactions occurring at very cathodic potentials in the melts. The effects of deposition parameters, such as deposition current density, on surface morphology and adherence of the deposits are evaluated. We are especially interested in the electrodeposition of aluminium on Al substrates at relatively high current densities (up to 200 mA/cm2) due to their practical importance. Our work aims to explore the possibility of using AlCl3-NaCl melts as potential electrolytes for the electrolytic extraction and recycling of aluminium in aluminium industry.
2 Experimental
All chemicals were handled in a dry argon-filled atmosphere glove box. The electrochemical experiments, including cyclic voltammetry and chronopotentiometry, were carried out using a three-electrode electrochemical cell and a PGSTAT 30 and a BOOSTER 20A. The electrolyte was AlCl3-NaCl melt system with 52?48 of molar ratio using continuous magnetic bar stirring, and the electrolyte temperature was controlled at 200 ℃ with silicone oil. The working electrode was tungsten (99.95%) wire of 1 mm in diameter. The counter electrode was a tungsten (99.95%) rod of 5 mm in diameter. The reference electrode was a high purity aluminium wire (99.999%) of 1 mm in diameter. To lower the ohmic drop, both reference and counter electrodes were directly immersed into the electrolytes. The distance between the Al tip of the reference electrode and the surface of working electrode was controlled to be about 5 mm. Prior to electrochemical measurements, the W working electrode and counter electrode were polished with increasingly finer grades of emery paper, followed consecutively by a polishing with alumina compounds down 0.05 μm. However, the Al reference electrode was polished further in a mixture of 25% (volume fraction) H2SO4 (98%), 70% H3PO4 (85%) and 5% HNO3 (52.5%) at room temperature for 10-15 min, to remove the residual oxide[5].
3 Results and discussion
3.1 Cyclic voltammetry analysis
Fig.1 shows a typical linear voltammogram recorded on the W electrode in AlCl3-NaCl melt system with 52?48 of molar ratio at 200 ℃. The AlCl4-, Al2Cl7- and Cl- concentrations in this electrolyte were reported to be 7.68 mol/L, 0.66 mol/L and 0.01 mmol/L, respectively[17].
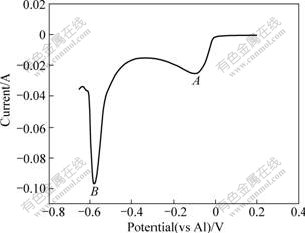
Fig.1 Linear voltammogram recorded on W electrode in AlCl3-NaCl melt system with 52?48 of molar ratio at 200 ℃ (v=0.1 V/s; S=0.636 cm2)
In this work, the W wire as the working electrode belongs to the column electrode, and length of 2 cm is far larger than radius (r=0.5 mm). So, for the diffusion mass transfer process, only the radial diffusion process is considered and linear diffusion process at the cross section of the working electrode may be neglected, which makes further discussion about diffusion current property more convenient[18]. Based on the above hypothesis, the relationship between current and potential is given as follows:
![]() (1)
(1)
where φ is the potential; B is the half-wave potential; n is the transfer electron number; T is the experimental temperature; R is the gas constant, 8.314 J/(K·mol); F is the Faraday constant, 96 500 C/mol; Ip is the maximum current; and I is the current.
According to Eq.(1) and data obtained from the linear voltammogram(shown in Fig.1), Fig.2 can be obtained. For the first reduction wave A, a plot of the potential, φ, versus ln[(Ip-I)/I] is linear (as shown in Fig.2(a)). The linear equation dealt with the least square method is given as follows:
![]() (2)
(2)
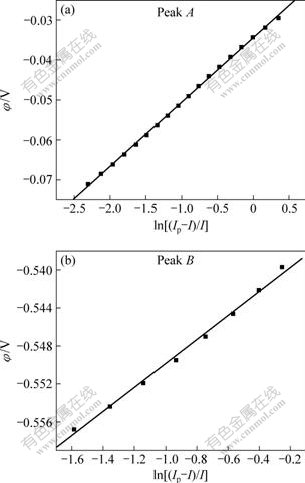
Fig.2 Potential φ as a function of ln[(Ip-I)/I]
The transfer electron number of n=2.8≈3 can be calculated from the slope of the potential φ versus ln[(Ip-I)/I] plot.
For the second reduction wave B,
![]() (3)
(3)
The transfer electron number of n=3.2≈3 can be calculated from the slope of the potential φ versus ln[(Ip-I)/I] plot (as shown in Fig.2(b)).
According to the above analysis, Al(Ⅲ) is reduced in two consecutive steps:
4Al2Cl7-+3e-→Al+7AlCl4- (4)
AlCl4-+3e-→Al+4Cl- (5)
Fig.3 shows a typical voltammogram recorded on W electrode in AlCl3-NaCl melt system with 52?48 of molar ratio at 200 ℃. The reduction of the relatively weak Al2Cl7- complex to aluminum (peak A) is seen to occur at a potential of about -0.016 V with respect to an aluminum reference electrode in the melt. One can see that aluminum deposition results in a localized acidity decrease near the electrode surface since Al2Cl7- is consumed and AlCl4- is produced. This is approximately 0.5 V positive of that required for the reduction of the more stable AlCl4- tetrahedral complex (peak B). When the potential sweep is reversed, two anodic peaks (A′, B′), both associated with the stripping of Al, are seen. The first stripping, occurring at about -0.45 V, is the anodic dissolution of metallic aluminum to form AlCl4 with the presence of Cl-. The second stripping wave occurs at about 0.15 V. The process at +2.476 V (peak C) results from the oxidation of the AlCl4- anions:
4AlCl4-→2Al2Cl7-+Cl2↑+2e- (6)
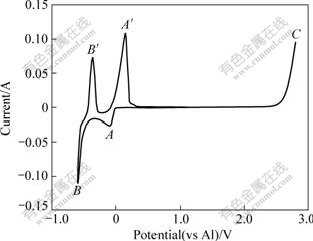
Fig.3 Cyclic voltammogram recorded on W electrode in AlCl3-NaCl melt with 52?48 of molar ratio at 200 ℃(cathodic and anodic limits are set to be -0.60 and +2.8 V, respectively; scan rate v=0.1 V/s; S=0.636 cm2)
Fig.4 and Fig.5 show a series of cyclic voltammograms on W electrode within different cathodic limits. To obtain more details on the aluminum electrodeposition, our measurements were focused on the potential between -0.2 V and +0.1 V, where the reduction of AlCl4- anions (peak B) and the oxidation of AlCl4- anions (peak C) can be avoided.
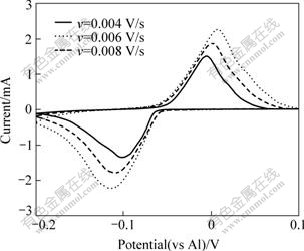
Fig.4 Cyclic voltammograms as function of scan rate recorded on W electrode in AlCl3-NaCl melt with 52?48 of molar ratio at 200 ℃ (cathodic and anodic limits are set to be -0.2 and +0.1 V, respectively; S=0.636 cm2)
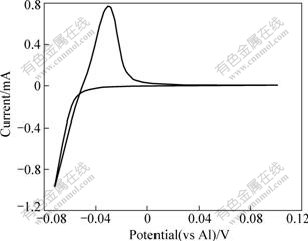
Fig.5 Cyclic voltammogram recorded on W electrode in AlCl3-NaCl melt with 52?48 of molar ratio at 200 ℃ (cathodic and anodic limits are set to be -0.07 and +0.1 V, respectively; v=0.01 V/s; S=0.636 cm2)
Fig.4 shows the linear sweep voltammograms performed at different sweep rates. The magnitude of the Al2Cl7- and AlCl4- reduction wave (Ip) is seen to increase linearly with the square root of the sweep rate (v1/2) in Fig.6. This is a clear indication that the reduction is limited by the diffusion of Al2Cl7- to the electrode and the electrochemical reactions corresponding to wave are reversible.
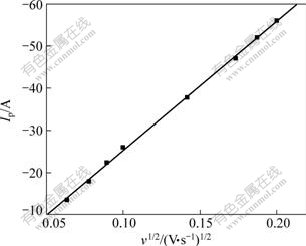
Fig.6 Peak current (Ip) as function of square root of scan rate (v1/2)
Fig.5 shows that certain nucleation overpotential was required. When the cathodic limit was set to be -0.07 V, the voltammogram exhibited a current loop hysteresis, typical of the deposition process requiring nucleation overpotential. This current loop occurs because the deposition of aluminum on W electrode during the negative scan requires a considerable overpotential in order to initiate the nucleation and subsequent growth of aluminum deposit.
Fig.7 shows the dependence of the coulombic efficiency (ratio of the stripping charge to deposition charge) with cathodic peak current from various scan rates. The coulombic efficiency initially increased with current density but decreased at higher current densities. This agrees with that reported in other molten salt[5]. The stripping of aluminium from this electrode proceeded with an average 86.8% of efficiency, resulting from the chemical corrosion reaction between the deposited aluminium and the melt or its impurities and the reduction of some electroactive impurities.
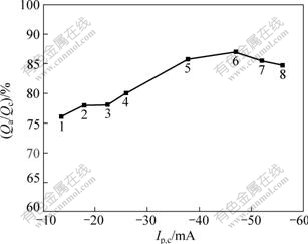
Fig.7 Coulombic efficiency of aluminum electrodeposition as function of current in AlCl3-NaCl melt system with 52?48 of molar ratio at 200 ℃ at different scan rates (S=0.636 cm2): 1—0.004 V/s; 2—0.006 V/s; 3—0.008 V/s; 4—0.01 V/s; 5—0.02 V/s; 6—0.03 V/s; 7—0.035 V/s; 8—0.04 V/s
3.2 Chronopotentiometry analysis
Aluminum deposition has also been studied by examining the time-dependent electrode potential under galvanostatic conditions. A typical result given in Fig.8 shows an initial sharp drop of the potential associated with nucleation of aluminum from reduction of Al2Cl7-. This is followed by relaxation to a plateau A (about -0.1 V) associated with the growth of the aluminum deposit. After a time interval, which is a function of the applied current (plateau A from Fig.8(a) and 8(b)), the potential begins to decrease with nucleation of aluminum from reduction of AlCl4-. This is followed by relaxation to a plateau B (about -0.52 V) associated with the growth of the aluminum deposit. When the current is reverse, plateau A′ and plateau B′ were observed due to the stripping of aluminum. This is in reasonable agreement with cyclic voltammograms (Fig.3). When the current given is less than 0.04 A, there is only nucleation and growth of aluminum from reduction of Al2Cl7-.
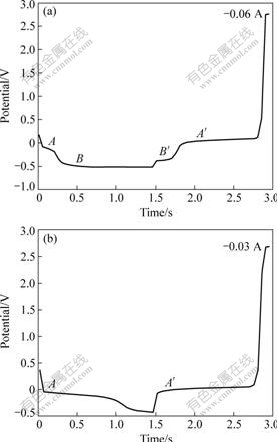
Fig.8 Chronopotentiometry recorded on W electrode in AlCl3-NaCl melt system with 52?48 of molar ratio at 200 ℃
3.3 Constant current deposition of aluminium
Constant current deposition of aluminium was conducted on both Al and Cu substrates in AlCl3-NaCl melt system with 52?48 of molar ratio. The electrolyte temperature was controlled at 200 ℃, so that higher deposition rate can be expected. The current density was varied between 50 and 200 mA/cm2 to study its effect on the surface morphology. Generally, the deposits obtained at lower current densities (50-100 mA/cm2) are quite dense and well adherent to the Al and Cu substrates. Those obtained current densities at above 200 mA/cm2 are found to have intergranular crevices growth with relatively poor adherence. Fig.9 and Fig.10 show the surface morphologies of the representative aluminium deposits respectively obtained at 70, 100, 200 mA/cm2 on Al substrate (Fig.9) and 100 mA/cm2 on Cu substrate (Fig.10(a)). The inset image in Fig.10(a) is the higher magnification micrographs. With a current density less than 100 mA/cm2, the sample substrate was covered with discontinuous aluminium deposits consisting of aluminium crystallites. Each of the aluminium crystallites consists of large amounts of small grains. The deposits obtained at 70 and 100 mA/cm2 are quite smooth and have similar surface morphology (Figs.9(a), (b)). The deposit obtained at 100 mA/cm2 is rougher, but it is still quite dense and well adherent (Fig.9(b)). The deposits obtained at 200 mA/cm2 or above 200 mA/cm2 are found to have intergranular crevices growth with relatively poor adherence (Fig.9(c)). It is possible to use AlCl3-NaCl melt as potential electrolytes for the electrolytic extraction or recycling of aluminium at a current density lower than 200 mA/cm2.
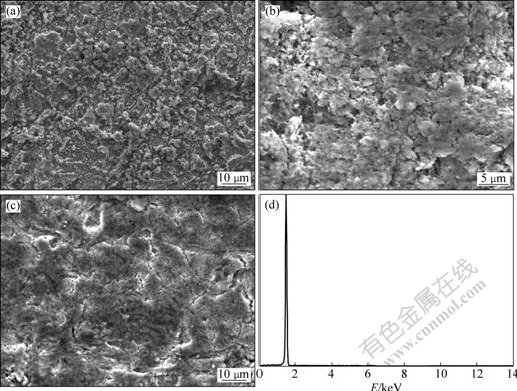
Fig.9 SEM images and XRD patterns of aluminium electrodeposits obtained on Al substrate from 52?48 molar ratio AlCl3-NaCl melt at 200 ℃ with different current densities for 1 h: (a) SEM image, 70 mA/cm2; (b) SEM image, 100 mA/cm2; (c) SEM image, 200 mA/cm2; (d) EDS analysis, 70 mA/cm2
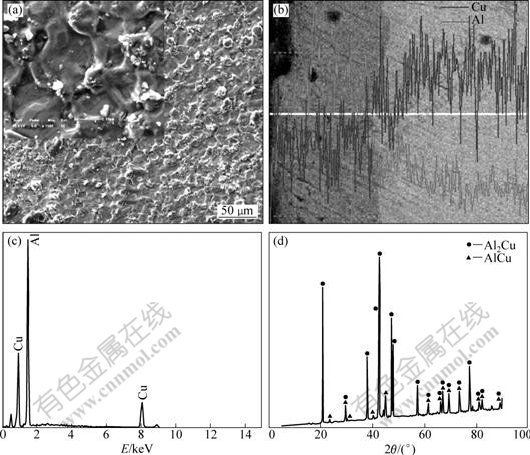
Fig.10 SEM micrographs and XRD patterns of aluminium electrodeposits obtained on Cu substrate from 52?48 molar ratio AlCl3-NaCl melt at 200 ℃ with different current densities for 1 h: (a) SEM image, 70 mA/cm2; (b) Linear scan image of side of sample; (c) EDS analysis, 70 mA/cm2; (d) XRD pattern, 70 mA/cm2
The electrochemical deposition of aluminum on copper electrode in AlCl3-NaCl melts indicates that intermetallic compounds are formed. A typical result is given in Fig.10(b). XRD results (Fig.10(d)) proved that the intermetallic compounds are AlCu and Al2Cu. The thickness of electrodeposition is about 20 μm.
4 Conclusions
1) The voltammetric studies show the electrochemical reaction of 4Al2Cl7-+3e-→Al+7AlCl4- is reversible. Certain nucleation overpotential was required during the deposition of aluminum on W electrode from AlCl3-NaCl melt with 52:48 molar ratio at 200 ℃. Chronopotentiometry analysis shows that Al (Ⅲ) is reduced in two consecutive steps under certain current density. This is in reasonable agreement with cyclic voltammograms.
2) By using constant current deposition, the electrodeposits on Al substrates obtained between 50 and 100 mA/cm2 are quite dense and well adherent to the Al substrate. Those obtained at a current densities higher than 200 mA/cm2 have intergranular crevices growth with relatively poor adherence. Our studies show that AlCl3-NaCl melt system can be possibly used as potential electrolytes for the electrolytic extraction and recycling of aluminium.
3) The electrochemical deposition of aluminum on copper electrode in AlCl3-NaCl melt indicates that intermetallic compounds are formed. The intermetallic compounds are AlCu and Al2Cu.
References
[1] ZHAO Y, VANDERNOOT T J. Electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts [J]. Electrochim Acta, 1997, 42(1): 3-13.
[2] BUZZEO M C, EVANS R G, COMPTON R G. Non-haloaluminate room-temperature ionic liquids in electrochemistry—A review [J]. Chem Phys Chem, 2004, 5(8): 1106-1120.
[3] ABBOTT A P, MCKENZIE K J. Application of ionic liquids to the electrodeposition of metals [J]. Phys Chem Chem Phys, 2006,8: 4265-4279.
[4] KAMAVARAM V, MANTHA D, REDDY R G. Recycling of aluminum metal matrix composite using ionic liquids: Effect of process variables on current efficiency and deposit characteristics [J]. Electrochim Acta, 2005, 50(16/17): 3286-3295.
[5] ZHAO Y, VANDERNOOT T J. Electrodeposition of aluminium from room temperature AlCl3-TMPAC molten salts [J]. Electrochim Acta, 1997, 42(11): 1639-1643.
[6] LEE J J, MILLER B, SHI X, KALISH R, WHEELER K A. Aluminium deposition and nucleation on nitrogen-incororated tetrahedral amorphous carbon electrodes in ambient temperature chloroaluminate melts [J]. J Electrochem Soc, 2000, 147(9): 3370-3376.
[7] ZHANG Li-peng, YU Xian-jin, HONG Shu-cui, DONG Yu-hui, LI De-gang, ZHANG Xin. Preparation and electrodeposition of Al-based ionic liquid (BMIM)Br-AlCl3 [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(S): 274-278. (in Chinese)
[8] JIANG T, CHOLLIER BRYM M J, DUB? G, LASIA A, BRISARD G M. Electrodeposition of aluminium from ionic liquids: Part I—electrodeposition and surface morphology of aluminium from aluminium chloride (AlCl3)–1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) ionic liquids [J]. Surface and Coatings Technology, 2006, 201: 1-9.
[9] JIANG T, CHOLLIER BRYM M J, DUB? G, LASIA A, BRISARD G M. Electrodeposition of aluminium from ionic liquids: Part Ⅱ—studies on the electrodeposition of aluminum from aluminum chloride (AlCl3)-trimethylphenylammonium chloride (TMPAC) ionic liquids [J]. Surface and Coatings Technology, 2006, 201: 10-18.
[10] LI Qing-feng, QIU Zhu-xian. Electrochemical deposition of aluminium from NaCl-AlCl3 molten salt system [J]. Rare Metal Materials and Engineering, 1995, 24(3): 59-63. (in Chinese)
[11] ROLLAND P, MAMANTOV G. Electrochemical reduction of Al2Cl7- ions in chloroaluminate molts [J]. J Electrochem Soc, 1976, 123: 1299-1303.
[12] NAYAK B, MISRA M M. The electrodeposition of aluminum on brass from a molten aluminum chloride-sodium chloride bath [J]. J Appl Electrochem, 1977, 7: 45-50.
[13] JAFARIAN M, MAHJANI M G, GOBAL F, DANAEE I. Effect of potential on the early stage of nucleation and growth during aluminum electrocrystallization from molten salt (AlCl3-NaCl-KCl) [J]. J Electroanal Chem, 2006, 588: 190-196.
[14] LI Qing-feng, HJULER H A, BERG R W, BJERRUM N J. Influence of substrates on the electrochemical deposition and dissolution of aluminum in NaAlCl4 melts [J]. J Electrochem Soc, 1991, 138: 763-766.
[15] LI Qing-feng, HJULER H A, BERG R W, BJERRUM N J. Electrochemical deposition of aluminium from NaCl-AlCl3 melts [J]. J Electrochem Soc, 1990, 137: 593-597.
[16] WANG Zhao-wen, KAN Hong-min, SHI Zhong-ning, GAO Bing-liang, BAN Yun-gang, HU Xian-wei. Electrochemical deposition and nucleation of aluminum ontungsten in aluminum chloride-sodium chloride melts [J]. J Mater Sci Technol, 2008, 24(6): 915-920.
[17] BOXALL L G, JONES H L, OSTERYOUNG R A. Solvent equilibria of AlCl3-NaCl melts [J]. J Electrochem Soc, 1973, 120(2): 223-231.
[18] LI Jun, LI Bing. Cathode process of titanium diboride electrodeposition in LiF-NaF-KF-K2TiF6-KBF4 molten salt [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(1): 1070-1075. (in Chinese)
Foundation item: Project(50672060) supported by the National Natural Science Foundation of China; Project(2007CB210305) supported by the National Basic Research Program of China
Corresponding author: KAN Hong-min; Tel: +86-13889305238; E-mail: kanhongmin2002@163.com
DOI: 10.1016/S1003-6326(09)60114-X
(Edited by YANG Hua)

