采用基于甲基丙烯酸羟乙酯-叔丁醇的凝胶注模体系制备多孔氧化铝陶瓷
来源期刊:中国有色金属学报(英文版)2019年第8期
论文作者:王小锋 谢雨洲 彭超群 王日初 张斗 冯艳
文章页码:1714 - 1720
关键词:凝胶注模;聚合;多孔陶瓷;氧化铝;甲基丙烯酸羟乙酯(HEMA);叔丁醇(TBA);弯曲强度
Key words:gelcasting; polymerization; porous ceramic; alumina; 2-hydroxyethyl methacrylate (HEMA); tert-butyl alcohol (TBA); bending strength
摘 要:为了获得高强度的多孔氧化铝陶瓷,研发一种基于甲基丙烯酸羟乙酯-叔丁醇(HEMA-TBA)的新型凝胶注模体系。采用流变仪、TG-DSC、SEM和弯曲强度测试等手段分别研究HEMA-TBA 凝胶注模体系的聚合、坯体的热分解行为、烧结体的显微组织和力学性能。结果表明:(1) 25 °C 时,适合该体系聚合的引发剂(过氧苯甲酰)的优化加入量为10 mg/mL;(2) 含HEMA-TBA凝胶注模体系的氧化铝悬浮液表现为剪切变稀流变行为,且其黏度足够低至凝胶注模工艺要求;(3) 多孔氧化铝试样的孔隙度为42%~56%,其相应的弯曲强度为(8±0.5)~ (91±4.5) MPa。
Abstract: To obtain porous alumina ceramic with high strength, a novel gelcasting system based on 2-hydroxyethyl methacrylate (HEMA) dissolved in tert-butyl alcohol (TBA) was developed. The polymerization of the HEMA-TBA gelcasting system, the thermal behavior of obtained green body, and the microstructures and mechanical properties of the sintered bodies were investigated by rheometer, TG-DSC, SEM and bending strength testing, respectively. The results show that, (1) 10 mg/mL of the initiator (benzoyl peroxide) is the optimal amount for polymerization of this gelscasting system at 25 °C; (2) The alumina suspension of the HEMA-TBA gelcasting system showing shear-thinning behavior is sufficiently low for gelcasting process; (3) The bending strength of porous alumina ceramic samples, whose porosities range from 42% to 56%, is from (8±0.5) to (91±4.5) MPa.
Trans. Nonferrous Met. Soc. China 29(2019) 1714-1720
Xiao-feng WANG1,2, Yu-zhou XIE2, Chao-qun PENG2, Ri-chu WANG2, Dou ZHANG1, Yan FENG2
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 2 August 2018; accepted 14 January 2019
Abstract: To obtain porous alumina ceramic with high strength, a novel gelcasting system based on 2-hydroxyethyl methacrylate (HEMA) dissolved in tert-butyl alcohol (TBA) was developed. The polymerization of the HEMA-TBA gelcasting system, the thermal behavior of obtained green body, and the microstructures and mechanical properties of the sintered bodies were investigated by rheometer, TG-DSC, SEM and bending strength testing, respectively. The results show that, (1) 10 mg/mL of the initiator (benzoyl peroxide) is the optimal amount for polymerization of this gelscasting system at 25 °C; (2) The alumina suspension of the HEMA-TBA gelcasting system showing shear-thinning behavior is sufficiently low for gelcasting process; (3) The bending strength of porous alumina ceramic samples, whose porosities range from 42% to 56%, is from (8±0.5) to (91±4.5) MPa.
Key words: gelcasting; polymerization; porous ceramic; alumina; 2-hydroxyethyl methacrylate (HEMA); tert-butyl alcohol (TBA); bending strength
1 Introduction
Gelcasting, firstly developed at Oak Ridge National Laboratory (ORNL) by OMATETE et al [1] in 1991, is a fabrication method in which ceramic particles are immobilized in three-dimensional networks resulted from the polymerization of organic monomers and cross-linkers, which are dissolved previously in the stabilized suspensions of these particles and triggered to polymerise by an initiator and a catalyst. In the past two decades, this method has undergone tremendous development. The gelcasting technology, initially invented for the fabrication of dense ceramics and widely used in the industry [2], is now also used for fabricating porous ceramic materials [3-6]. However, there is a stone blocking the development of gelcasting for porous ceramic, i.e., large shrinkage of green-body during drying, resulting in rapidly decreasing its porosity, which stems from tension force of solvent dragging the particles in green-body closer to each other [7,8].
To solve this problem, CHEN et al [7] developed a gelcasting system with tert-butyl alcohol (TBA) as the solvent, acrylamide (AM) as the monomer, and methylene-bis-acrylamide (MBAM) as the cross-linker. The advantage of this system is the low shrinkage of the green body during drying due to the high saturation vapor pressure and the low surface tension of TBA. This gelcasting system has been utilized to fabricate porous ceramics such as ZrO2 [9,10], hydroxyapatite (HAP) [11], and porous ceramic composites [12]. However, this gelcasting system still has several problems. Firstly, the initiator, (NH4)2S2O8, is insoluble in TBA, and the ionization of (NH4)2S2O8 is limited in TBA. Therefore, a large amount of (NH4)2S2O8 must be added to initiate the polymerization of monomers, and the rate of polymerization is low [13]. Secondly, the polymeric product of this system, polyacrylamide (PAM), is also insoluble in TBA, resulting in uneven and low compression strength of the green body. Lastly, the monomer of this system, acrylamide, is a neurotoxin, which limits the application range of the acrylamide system [3,14].
Herein, we have developed a gelcasting system for porous ceramic, which consists of tert-butyl alcohol (TBA) as the solvent, 2-hydroxyethyl methacrylate (CH2=C(CH3)COOCH2CH2OH, HEMA) as the monomer, methylene-bis-acrylamide (MBAM) as the cross-linker, benzoyl peroxide (BPO) as the initiator, and N,N-dimethylaniline (DMA) as the catalyst. In fact, HEMA usually used for lens materials is a low-toxic and widely used reagent. It had been used as a monomer to fabricate dense ceramic [14,15] and glass [16]. However, the water was taken as a solvent in these works and the properties of the system containing HEMA, e.g., polymerization, have not been reported until now.
In this work, the feasibility of the HEMA-TBA gelcasting system for porous ceramic was evaluated. The polymerization of the gelcasting system in TBA, rheological properties of alumina-TBA suspensions, and properties of the sintered body were investigated. Furthermore, porous alumina ceramic was produced using the HEMA-TBA gelcasting system.
2 Experimental
2.1 Materials
Alumina (Al2O3) powder with an average particle size of 1 μm was obtained from Henan Jiyuan Brother Material Co., China. The solvent, tert-butyl alcohol (TBA), was from Shanghai Chemical Reagent Co., China. To produce stable alumina-TBA suspensions, citric acid (Shanghai Chemical Reagent Co., Shanghai, China) was used as a dispersant. 2-hydroxyethyl methacrylate (HEMA; Shanghai Chemical Reagent Co., Shanghai, China), as a monomer, and methylene-bis- acrylamide (MBAM), as a cross-linker, were dissolved in TBA as premix solutions. The mass ratio of TBA/HEMA/MBAM was 25:5:1. Benzoyl peroxide (BPO; Shanghai Chemical Reagent Co., Shanghai, China) as an initiator and N, N-dimethylaniline (DMA; Shanghai Chemical Reagent Co., Shanghai, China) as a catalyst were used to initiate polymerization. All these materials were of analytical reagent (AR) grade.
2.2 Methods
To study the polymerization of the HEMA-TBA gelcasting system, HEMA and MBAM were dissolved in TBA with a mass ratio of 5:1, and the concentration of HEMA was 20 wt.% of the solvent. The prepared premix solutions were solidified by adding initiator at various concentrations (5, 10, 15 and 20 mg/mL). The mass ratio of initiator to catalyst is fixed as 2:1, and the testing temperature was fixed at 25 °C. In addition, to detect the polymerization kinetics of the HEMA-TBA gelcasting system, the prepared premix solutions were solidified at various temperatures (15, 25, 35 and 45 °C), where the amounts of initiator and catalyst were 5 and 2.5 wt.% of the monomer, respectively.
A homogeneous suspension of the Al2O3 powder with HEMA and MBAM in TBA was prepared by adding citric acid (2 wt.% of powder) and ball milling for 24 h. The solid loadings of the prepared suspensions were 5%, 10%, 15% and 20%. The initiator and catalyst were then added to the suspension, following which it was poured into polyethylene molds. The amounts of initiator and catalyst were 5 and 2.5 wt.% of the monomer, respectively. The solid product was removed from the mold and dried at 40 °C for 48 h. The green body was then heated at 450 °C for 1 h to remove the polymer and subsequently sintered at 1500 °C for 1 h.
2.3 Characterization
A stress-controlled rheometer (AR2000, TA Instruments, New Castle, PA, USA) with a parallel plate (40 mm in diameter) was used to study the rheological properties of Al2O3 suspension prepared with or without the HEMA-TBA gelcasting system and to measure the storage modulus (G′) of premix solution for detecting the polymerization of the HEMA system. The testing conditions were set based on our previous work [11]. The rheological properties were measured in the steady-state mode with a shear rate ranging from 1 to 1000 s-1 at 25 °C. The oscillatory mode with a frequency of 1 Hz and a strain of 0.4% was used to study the polymerization of the HEMA system.
Thermogravimetric differential scanning calorimetry (TG-DSC) measurement was performed using a differential scanning calorimeter (DSC 200 F3 Maia, NETZSCH Instruments Manufacturing Co., Selb, Germany) for a dried green alumina body with 15% solid loading. The thermally activated decomposition of the gelcast polymers was investigated in the air with a constant heating rate of 1 °C/min from room temperature to 1200 °C.
The porosity of the sintered body was measured using the Archimedes method. A dynamic testing machine (MTS 810, MTS Systems Co., Shanghai, China) was used to study the room-temperature mechanical strength of the sintered body with a size of 4 mm in width, 3 mm in height, and 45 mm in length. The crosshead speed in flexural tests was 0.5 mm/min. Scanning electron microscopy (SEM, Quanta-200, FEI, Eindhoven, Netherlands) was used to observe the microstructures of the sample.
3 Results and discussion
3.1 Polymerization kinetics
In gelcasting process, a suitable gelation rate is one of the most critical factors for the successful production of ceramics [14]. The initiator concentration and temperature have been regarded as two of the most important parameters that determine the gelation rate [17]. It has been revealed that HEMA in a water- based system undergoes a typical free-radical polymerization process similar to the polymerization of the acrylamide system. Before the gelation, the storage modulus (G′) and viscosity of the suspension were very low and remained constant. When gelation occurred in the reaction period, the suspension transformed into a rigid gel accompanied by a sharp increase of G′. In general, an induction time of 5-120 min was considered reasonable.
The polymerization of the HEMA system was studied using the oscillatory shear deformation method to monitor the change of G′. Figure 1 shows the storage modulus of a premix solution of 20 wt.% HEMA and 4 wt.% MBAM with different initiator additions as a function of time at 25 °C, where the mass ratio of the initiator to catalyst was 2:1. It can be seen from Fig. 1 that polymerization did not occur when the amount of initiator was 5 mg/mL. When the amount of initiator was in the range of 10-15 mg/mL, the induction time decreased slightly with the increase of initiator amount. When the amount of initiator increased to 20 mg/mL, the induction time increased to 920 s. For initiator concentration in the range of 10-20 mg/mL, the induction time was approximately 500-1000 s, which is suitable for gelcasting. In addition, the storage modulus decreased with increasing indicator amount.

Fig. 1 Storage modulus (G′) of premix solution of HEMA- TBA gelcasting system with different initiator concentrations (5-20 mg/mL) during gelation at 25 °C
According to the theory of organic chemistry, the induction period includes two steps [18]. In the first step, the initiator is decomposed to two primary radicals:
R-R→2R· (1)
The second step is the reaction of the monomer and primary radical:
R·+CH2=CH2→RCH2CH2· (2)
The first step is a slow endothermic reaction, while the second step is a fast exothermic reaction. Therefore, the induction time depends on the reaction rate of the first step. However, some side reactions such as induced decomposition and caged reaction, which consume the primary radical, result in an increase of induction time. In Fig. 1, when the initiator amount was 5 mg/mL, most of the primary radical was consumed by side reactions, and the remnant primary radical was insufficient to initiate the reaction. With the increase of initiator amount, the induction time should decrease. However, the result in Fig. 1 is inconsistent with this rule. This inconsistency might be due to consumption of the primary radical by the excessive DMA, but the exact mechanism is not clear.
Temperature is one of the most important factors determining the induction time of gelcasting. According to the theory of organic chemistry, a high reaction temperature results in the rapid decomposition of the initiator to free radicals, which is the step that determines the induction time. Because TBA is a volatile solvent, the reaction temperature should not be too high. However, a low reaction temperature will result in a long induction time, which is ineffective, inconvenient, and expensive. Therefore, the temperature should be moderate. In general, a reaction temperature range of 15-45 °C is suitable.
Figure 2 shows the storage modulus of a premix solution of 20 wt.% HEMA and 5 wt.% MBAM with constant initiator additions as a function of time. It is clear that a higher temperature results in a shorter induction time, which is in agreement with previous studies on free-radical polymerization [18,19]. For a reaction temperature of 15 °C, the induction time is approximately 1400 s, and the storage modulus increases with time after the induction period. When the reaction temperature is increased to 25 °C, the induction time significantly decreases to 650 s. As the temperature is increased further, the induction time continues to decrease, but the decreasing tendency becomes relatively small. Therefore, a reaction temperature of 25 °C is most suitable. In addition, the storage modulus of the polymerized pure gel slightly decreases with increasing reaction temperature. The relationship of induction time (ti) and temperature (T) can be expressed by an Arrhenius-type equation:
 (3)
(3)
where r is the reaction rate, R is the Avogadro constant, and Ea is the activation energy of the HEMA polymerization reaction. Figure 3 shows the Arrhenius plot data from Fig. 2. From the fitting line, it is revealed as expected that the logarithm of induction time is inversely proportional to the temperature. The calculated Ea is 40.7 kJ/mol, which is similar to that of the acrylamide system (79.4 [20], and 71.2 kJ/mol [21]). Therefore, the HEMA gelcasting is controllable in the temperature range of 15-45 °C.

Fig. 2 Storage modulus (G′) of premix solution of HEMA- TBA gelcasting system with initiator concentration of 10 mg/mL during gelation at low temperature (15-45 °C)

Fig. 3 Arrhenius plot of data calculated from Fig. 2
3.2 Fluidity of suspensions
The most important factor for successful production with the gelcasting process is the realization of a stable suspension with low viscosity and high solid loading. The viscosity of suspensions depends on many factors, such as the dispersant, solid loading, pH, and gelcasting system [21-24]. In a gelcasting system, this factor should be considered. Figure 4 shows the viscosity of the suspension as a function of shear rate for different solid loadings with dispersant and the HEMA system. For comparison, the viscosity curve for 15% solid loading with the only dispersant is also shown. As shown in Fig. 4, all the suspensions with or without the HEMA-TBA gelcasting system show similar shear- thinning behaviors, which reveals that the HEMA-TBA gelcasting system does not influence the rheological behavior of the suspension. In addition, the viscosity and shear stress of the suspension with 15% solid loading containing the HEMA-TBA gelcasting system are lower than those of the suspension without the HEMA-TBA gelcasting system. This indicates that the addition of the HEMA system can reduce the viscosity of the suspension, which is beneficial to the gelcasting process. The viscosity of these suspensions is less than that of suspensions produced with water-based gelcasting, and it is sufficiently low for the gelcasting process [15].

Fig. 4 Rheological behaviour of suspensions with different solid loadings as function of shear rate
The relationship between the viscosity and shear rate for suspensions with shear-thinning behavior can be described with the empirical Ostwald-de Waele power-law model [25]:
η=Kγn-1 (4)
where η is the viscosity, γ is the shear rate, n is the power-law index, and K is the consistency index. The parameters of the Ostwald-de Waele line fitting in Fig. 4 are listed in Table 1, in which the values of the power-law index n with different solid loadings is less than 1, confirming that the suspensions display a similar behavior of shear-thinning. This result suggests that the HEMA-TBA gelcasting system does not influence the rheological behaviour of the suspension.
3.3 Consolidation of suspensions
The consolidation of suspensions is an important step of gelcasting. It depends on the properties of the green body. If the rate of solidification is too high, the suspension cannot fill the mold, which leads to the defects of the green body. On the other hand, it is inconvenient and expensive to adopt a process with an excessively low rate of solidification. Figure 5 shows the storage modulus as a function of time for suspensions with different solids after adding 10 mg/mL initiator and 5 mg/mL catalyst at 25 °C. For reference, the storage modulus as a function of time for the premix solution without powder is also shown in Fig. 5. It can be seen that the storage modulus at the beginning of the measurement increases with increasing solid loading. However, these values of storage modulus are too low to keep a fixed shape, and it exceeds 104 Pa after 30 min, which is sufficiently high for gelcasting [20]. In addition, the induction period decreases with increasing solid loading. The induction time of suspensions with different solid loadings is suitable for gelcasting. The induction period of the premix solution without powder is approximately 680 s, while the induction period of the suspensions with 20% solid loading is approximately 300 s. It is worth noting that G′ increases at the beginning of the measurement. This can be attributed to the fact that the increase of interaction between particles and polymer molecules hinders the diffusion of free-radicals, which results in the high concentration of free-radicals at parts of suspensions [20,26].
Table 1 Parameters of Ostwald-de Waele line fitting for alumina suspensions with different solid loadings in Fig. 4


Fig. 5 Storage modulus of alumina suspensions containing HEMA-TBA gelcasting system as function of time
3.4 Binder burnout investigation
The debinding process depends on the pyrolysis of the polymerized HEMA in the alumina green body during sintering. The TG and DSC curves in Fig. 6 show that the green body of the HEMA-TBA gelcasting system has two main processes involving mass loss and one exothermic peak. One process involving mass loss occurs in the temperature range of 25-180 °C, where the DSC curve has a small endothermic peak because the remaining TBA volatilizes. The other process involving mass loss with an exothermic peak occurs in the temperature range of 250-420 °C, which should be ascribed to the decomposition and burnout of the polymerized HEMA. Compared to polymerized AM, polymerized HEMA has lower decomposition and burnout temperatures, which implies that polymerized HEMA may be removed more easily than PAM [15]. According to the TG-DSC curves, the debinding process lasts for 1 h at a constant temperature of 450 °C, which is sufficient to remove the organics in the green body.
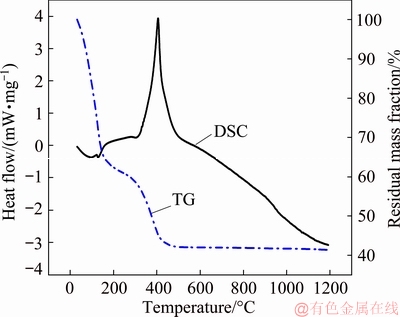
Fig. 6 TG-DSC curves of alumina green body of HEMA-TBA gelcasting system
3.5 Microstructure and mechanical properties
The properties of the produced porous ceramic are dependent on the sizes, amounts, shapes, locations, and connectivity of distributed pores. Figures 7(a-d) show the microstructures of porous alumina ceramic sintered at 1500 °C with different solid loadings ranging from 5% to 20%. As can be seen, the pore size decreases with increasing solid loading. In the microstructure, pores with irregular shapes uniformly distribute with hardly any defect. The porosity and mechanical properties of the sintered body are listed in Table 2. It is demonstrated that a higher solid loading results in lower porosity and higher bending strength. As the solid loading increases from 5% to 20%, the porosity decreases from 56% to 42%, and the bending strength increases from (8±0.5) to (91±4.5) MPa. The results indicate that the highly porous alumina ceramic prepared using the HEMA-TBA gelcasting system possesses relatively high mechanical strength.

Fig. 7 Microstructures of porous alumina ceramic with different solid loadings
Table 2 Porosity and bending strength of porous ceramics with different solid loadings

4 Conclusions
(1) For a premix solution of 20 wt.% HEMA and 4 wt.% MBAM in TBA, it was confirmed that 10 mg/mL of the initiator is optimal for the gelcasting processing.
(2) Temperature has a significant effect on the induction time of this gelcasting system, and 25 °C is determined to be most suitable. Similar to the conventional aqueous acrylamide gelcasting system, the relationship between induction time and reaction temperature of HEMA-TBA system satisfies the Arrhenius-type equation, i.e., the induction time decreases with increasing temperature.
(3) The rheological behaviors of suspensions with and without the HEMA-TBA gelcasting system are similar (shear-thinning behavior), but the suspensions with the HEMA-TBA gelcasting system have lower viscosity.
(4) The induction time of polymerization decreases with increasing solid loading because the diffusion of free-radicals is hindered.
(5) The decomposition and burnout temperature of polymerized HEMA-MBAM is around 420 °C.
(6) Porous alumina ceramic with 42%-56% porosity and a bending strength of ((8±0.5)-(91±4.5)) MPa has been successfully produced.
Acknowledgments
This work was supported by State Key Laboratory of Powder Metallurgy, Central South University, Changsha, China.
References
[1] OMATETE O, JANNEY M, STREHLOW R. Gel casting: A new ceramic forming process [J]. American Ceramic Society Bulletin, 1991, 70(10): 1641-1649.
[2] OMATETE O, JANNEY M, NUNN S. Gelcasting: From laboratory development toward industrial production [J]. Journal of the European Ceramic Society, 1995, 17(2-3): 407-413.
[3] YANG J, YU J, HUANG Y. Recent developments in gelcasing of ceramics [J]. Journal of the European Ceramic Society, 2011, 31(14): 2569-2591.
[4] DENG X, WANG J, ZHANG H, LIU J, ZHAO W, HUANG Z, ZHANG S. Effects of firing temperature on the microstructures and properties of porous mullite ceramics prepared by foam-gelcasting [J]. Advances in Applied Ceramics, 2016, 115(4): 204-209.
[5] SALOMAO R, CARDOSO P, BRANDI J. Gelcasting porous alumina beads of tailored shape and porosity [J]. Ceramics International, 2014, 40(10): 16595-16601.
[6] BLACKBURN S. New processes or old: Complex shape processing of advanced ceramics [J]. Advances in Applied Ceramics, 2005, 104(3): 97-102.
[7] CHEN R, HUANG Y, WANG C, QI J. Ceramics with ultra-low density fabricated by gelcasting: An unconventional view [J]. Journal of the American Ceramic Society, 2007, 90(11): 3424-3429.
[8] WANG Xiao-feng, PENG Chao-qun, WANG Ri-chu, SUN Yue-hua, CHEN Yi-xin. Liquid drying of BeO gelcast green bodies using ethanol as liquid desiccant [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(7): 2466-2472.
[9] HU L F, WANG C A, HUANG Y. Porous yttria-stabilized zirconia ceramics with ultra-low thermal conductivity [J]. Journal of Materials Science, 2010, 45(12): 3242-3246.
[10] XU H, DU H, LIU J, GUO A. Preparation of sub-micron porous yttria-stabilized ceramics with ultra-low density by a TBA-based gel-casting method [J]. Chemical Engineering Journal, 2011, 173(1): 251-257.
[11] KIM J H, LEE J H, YANG T Y, YOON S, KIM B, PARK H. TBA-based freeze/gel casting of porous hydroxyapatite scaffolds [J]. Ceramics International, 2011, 37(7): 2317-2322.
[12] LEE J H, KIM W Y, YANG T Y, YOON S, KIM B, PARK H. Fabrication of porous ceramic composites with improved compressive strength from coal fly ash [J]. Advances in Applied Ceramics, 2011, 110(4): 244-250.
[13] LIU W. Fabrication and characterization of porous alumina ceramics with high porosity and high strength [D]. Beijing: Tsinghua University, 2009. (in Chinese)
[14] KOKABI M, BABALUO A, BARATI A. Gelation process in low-toxic gelcasting system [J]. Journal of the European Ceramic Society, 2006, 26(15): 3083-3090.
[15] CAI K, HUANG Y, YANG J. Alumina gelcasting by using HEMA system [J]. Journal of the European Ceramic Society, 2005, 25(7): 1089-1093.
[16] KOTZ F, ARNOLD K, BAUER W, SCHILD D, KELLER N, SACHSENHEIMER K, NARGANG T M, RICHTER C, HELMER D, RAPP B E. Three-dimensional printing of transparent fused silica glass [J]. Nature, 2017, 544: 337-339.
[17] ORTEGA F, SEPULVEDA P, PANDOLFELLI V. Monomer system for the gelcasting of foams [J]. Journal of the European Ceramic Society, 2002, 22(9): 1395-1401.
[18] MATYJASZEWSKI K, DAVIS T. Handbook of radical polymerization [M]. Hoboken: John Wiley & Sons, 2003.
[19] YOUNG A, OMATETE O, JANNEY M, MENCHHOFER P. Gelcasting of alumina [J]. Journal of the American Ceramic Society, 1991, 74(3): 612-618.
[20] WANG Xiao-feng, WANG Ri-chu, PENG Chao-qun, LUO Yu-lin, WANG Zi-yong. Gelation kinetics and uniformity of gelcasting [J]. Journal of Central South University, 2012, 43(4): 1281-1289. (in Chinese)
[21] LEWIS J. Colloidal processing of ceramic [J]. Journal of the American Ceramic Society, 2010, 83(10): 2341-2359.
[22] HE R, HU P, ZHANG X, ZHANG X, HAN W. Dispersion and Rheology of aqueous zirconium diboride nanosuspensions [J]. International Journal of Applied Ceramic Technology, 2014, 11(4): 706-713.
[23] MA J, LIN X, XIE Z, MIAO H, ZHANG B, CHENG Y. Gelcasting of alumina ceramic in mixed PVP–HEMA systems [J]. British Ceramic Transactions, 2004, 103(6): 257-260.
[24] HOU Z, DU H, LIU J, HAO R, DONG X, LIU M. Fabrication and properties of mullite fiber matrix porous ceramics by a TBA-based gelcasting process [J]. Journal of the European Ceramic Society, 2013, 33(4): 717-725.
[25] TADROS T F. Rheology of dispersions: principles and applications [M]. Weiheim: Weily-VCH, 2010.
[26] TALLON C, MORENO R, NIETO I, JACH D, ROKICKI G, SZAFRAN M. Gelcasting performance of alumina aqueous suspensions with glycerol monoacrylate: A new low-toxicity acrylic monomer[J]. Journal of the American Ceramic Society, 2010, 90(5): 1386-1393.
王小锋1,2,谢雨洲2,彭超群2,王日初2,张 斗1,冯 艳2
1. 中南大学 粉末冶金国家重点实验室,长沙 410083;
2. 中南大学 材料科学与工程学院,长沙 410083
摘 要:为了获得高强度的多孔氧化铝陶瓷,研发一种基于甲基丙烯酸羟乙酯-叔丁醇(HEMA-TBA)的新型凝胶注模体系。采用流变仪、TG-DSC、SEM和弯曲强度测试等手段分别研究HEMA-TBA 凝胶注模体系的聚合、坯体的热分解行为、烧结体的显微组织和力学性能。结果表明:(1) 25 °C 时,适合该体系聚合的引发剂(过氧苯甲酰)的优化加入量为10 mg/mL;(2) 含HEMA-TBA凝胶注模体系的氧化铝悬浮液表现为剪切变稀流变行为,且其黏度足够低至凝胶注模工艺要求;(3) 多孔氧化铝试样的孔隙度为42%~56%,其相应的弯曲强度为(8±0.5)~ (91±4.5) MPa。
关键词:凝胶注模;聚合;多孔陶瓷;氧化铝;甲基丙烯酸羟乙酯(HEMA);叔丁醇(TBA);弯曲强度
(Edited by Bing YANG)
Foundation item: Project (51202296) supported by the National Natural Science Foundation of China
Corresponding author: Yan FENG; Tel: +86-731-88836638; E-mail: 13467516329@163.com
DOI: 10.1016/S1003-6326(19)65078-8

