不同SnO2粒度制备的AgSnO2触头材料电弧侵蚀行为
来源期刊:中国有色金属学报(英文版)2016年第3期
论文作者:张苗 王献辉 杨晓红 邹军涛 梁淑华
文章页码:783 - 790
关键词:AgSnO2触头材料;SnO2粒度;电弧侵蚀;导电率;硬度
Key words:AgSnO2 contact materials; SnO2 particle size; arc erosion; electrical conductivity; hardness
摘 要:为了阐明SnO2粒度大小对AgSnO2触头材料电弧侵蚀行为的影响,采用粉末冶金法制备不同SnO2粒度的Ag-4%SnO2(质量分数)触头材料,对触头材料组织进行观察,并对其致密度、硬度和导电率进行测量。对Ag-4%SnO2触头材料进行电弧侵蚀实验,确定燃弧时间和电弧侵蚀前后的质量变化,并对电弧侵蚀后触头材料表面的形貌和成分进行表征。结果表明:细小的SnO2颗粒有助于提高Ag-4%SnO2触头材料的致密度和硬度,但降低了其导电率。随着SnO2粒度的减小,Ag-4%AgSnO2触头材料的燃弧时间变短,质量损失降低,电弧侵蚀面积增大,蚀坑变浅且分散。
Abstract: To clarify the effect of SnO2 particle size on the arc erosion behavior of AgSnO2 contact material, Ag-4%SnO2 (mass fraction) contact materials with different sizes of SnO2 particles were fabricated by powder metallurgy. The microstructure of Ag-4%SnO2 contact materials was characterized, and the relative density, hardness and electrical conductivity were measured. The arc erosion of Ag-4%SnO2 contact materials was tested, the arc duration and mass loss before and after arc erosion were determined, the surface morphologies and compositions of Ag-4%SnO2 contact materials after arc erosion were characterized, and the arc erosion mechanism of AgSnO2 contact materials was discussed. The results show that fine SnO2 particle is beneficial for the improvement of the relative density and hardness, but decreases the electrical conductivity. With the decrease of SnO2 particle size, Ag-4%SnO2 contact material presents shorter arc duration, less mass loss, larger erosion area and shallower arc erosion pits.
Trans. Nonferrous Met. Soc. China 26(2016) 783-790
Miao ZHANG, Xian-hui WANG, Xiao-hong YANG, Jun-tao ZOU, Shu-hua LIANG
Shaanxi Key Laboratory of Electrical Materials and Infiltration Technology, Xi’an University of Technology, Xi’an 710048, China
Received 30 April 2015; accepted 12 October 2015
Abstract: To clarify the effect of SnO2 particle size on the arc erosion behavior of AgSnO2 contact material, Ag-4%SnO2 (mass fraction) contact materials with different sizes of SnO2 particles were fabricated by powder metallurgy. The microstructure of Ag-4%SnO2 contact materials was characterized, and the relative density, hardness and electrical conductivity were measured. The arc erosion of Ag-4%SnO2 contact materials was tested, the arc duration and mass loss before and after arc erosion were determined, the surface morphologies and compositions of Ag-4%SnO2 contact materials after arc erosion were characterized, and the arc erosion mechanism of AgSnO2 contact materials was discussed. The results show that fine SnO2 particle is beneficial for the improvement of the relative density and hardness, but decreases the electrical conductivity. With the decrease of SnO2 particle size, Ag-4%SnO2 contact material presents shorter arc duration, less mass loss, larger erosion area and shallower arc erosion pits.
Key words: AgSnO2 contact materials; SnO2 particle size; arc erosion; electrical conductivity; hardness
1 Introduction
The electrical contact materials are widely used in switches, relays, contactors and circuit breakers and their properties directly affect the stability and reliability of electric system [1-3]. Hence, it is required that the contact materials should have excellent electrical and thermal conductivities, erosion resistance, welding resistance and low contact resistance [4-6]. Though AgSnO2 contact material has shown a promising prospect due to its environmental benign and remarkable performances [7-12], the arc erosion mechanism of AgSnO2 contact material is still obscure so far. RIEDER and WEICHSLER [13] thought that the arc erosion is strongly influenced by the mechanical action. RONG [14] found out that the arc erosion depends on the surface dynamics of AgSnO2 material. BEHRENS et al [15] reported that the arc erosion resistance is correlated with the microstructure and mechanical strength of AgSnO2 material after arc erosion. DU et al [16] believed that the arc erosion depends on the thermodynamic properties of each component, the surface composition and microstructure, as well as the dynamic response of the molten pool to the arc. ZHU et al [17,18] revealed that the wettability of Ag and SnO2 plays an important role in the arc erosion behavior. LUNGU et al [19] and WANG et al [20] thought that the structure homogenization obviously affects the arc erosion resistance of AgSnO2 material. WITTER and CHEN [21] found out that the SnO2 content is of major importance for erosion resistance. SWINGLER and SUMPTION [22] reported that the erosion mechanism is related to the element species of the contact materials during the arc discharge. XU et al [23] found out that the input energy of the arc is crucial to the arc erosion of AgMeO contact materials in comparison with the MeO density, thermal conductivity and volume fraction of MeO. ZHANG et al [24] believed that the erosion behavior is the combined action of materials, circuit parameters and the service environment. LIN et al [25] reported the effect of SnO2 particle size on the microstructure and physical properties of AgSnO2 composites, while the arc erosion was not studied in their research. BRAUMANN and KOFFLER [26] thought that larger oxide particles can achieve equivalent or better switching performance of AgSnO2 contact materials whereas the reason why AgSnO2 contact material with larger oxide particles has a good arc erosion behavior is not clarified.
From the aforementioned study, it can get some understanding of the arc erosion mechanism of AgSnO2 contact material, but the effect of SnO2 particle size on the arc erosion behavior of AgSnO2 contact material is still unclear. As the properties depend on the microstructure, the SnO2 particle size inevitably affects the erosion behavior of AgSnO2 contact material. Consequently, it is of significance to gain deep insights of the effect of SnO2 particle size on the arc erosion behavior of AgSnO2 contact material. In the present work, Ag-4%SnO2 (mass fraction) contact materials with different SnO2 particle sizes were fabricated by powder metallurgy. The effect of SnO2 particle size on the microstructure and properties of Ag-4%SnO2 contact materials was studied, and the mechanism of arc erosion was discussed.
2 Experimental
Ag powder (purity≥99.9%, particle size of 73 μm) and SnO2 powder (purity≥99.8%, particle sizes of 300 and 800 nm) were used to fabricate AgSnO2 contact materials. The morphologies of the starting Ag powder and SnO2 powder were characterized by a JSM-6700F scanning electron microscope (Fig. 1), and the particle size distribution of the starting SnO2 powder was analyzed by a BT-2003 laser diffraction-based particle size analyzer (Fig. 2). The Ag and SnO2 powders with a mass ratio of 96:4 were mixed for 6 h in a custom-made vibrating mill containing agate balls at a mass ratio of ball to powder of 40:1 and a rotational speed of 200 r/min, and 1% (mass fraction) absolute ethyl alcohol was adopted as process control agent during milling. The relative density of green compact was pre-set as 75% by controlling the compact dimension and mass. The composite powder was compressed in a closed die on a TM-106 hydraulic press under a pressure of 300 MPa for 40 s to obtain a compact with a cylindrical shape, 15 mm in diameter and 7 mm in thickness, followed by sintering at 700 °C for 2 h under argon gas. The electrical conductivity and hardness were tested on a 7501 eddy electrical conductivity gauge and a HB-3000 Brinell hardness tester under the load of 2500 N holding for 30 s, respectively, and the values were the average of three measured results. The density was measured utilizing Archimedes method. The microstructure of Ag-4%SnO2 contact materials was examined by a GX71 Olympus microscope.
The arc erosion was tested in an arc extinguishing chamber modified by a TDR240A crystal furnace, and
the circuit of vacuum electrical breakdown is shown in Fig. 3. The sample as a cathode was put in a Cu platform, which can move up and down in the vacuum chamber. Above the cathode, there was a pure tungsten rod with a radius of 5 mm and a tip radius of 1 mm as the anode. When the chamber was evacuated to 5.0×10-3 Pa and the capacitor of 120 μF was charged at the voltage of 3.5 kV, the cathode moved upward at a velocity of 0.2 mm/min until the gap was broken down, and then the cathode moved back to its initial position, and the gap between cathode and anode was 0.5 mm. The operations were repeated 50 times. The arc duration can be collected from the discharged waveform recorded by a Tektronix TDS-2014 dual channel digital memory oscilloscope. The mass of Ag-4%SnO2 contact materials before and after arc erosion was measured by a TG328A photoelectric analytical balance, the surface morphologies and compositions of the eroded Ag-4%SnO2 contact materials were characterized by a JSM-6700F scanning electron microscope (SEM) equipped with an energy disperse spectroscope (EDS), and the erosion areas were calculated by an Image-Pro Plus 6.0 software.
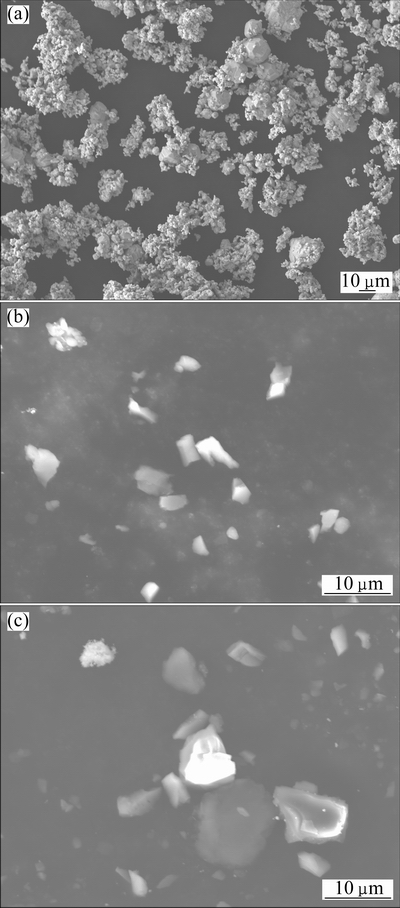
Fig. 1 Morphologies of starting Ag powder (a), 300 nm SnO2 powder (b) and 800 nm SnO2 powder (c)
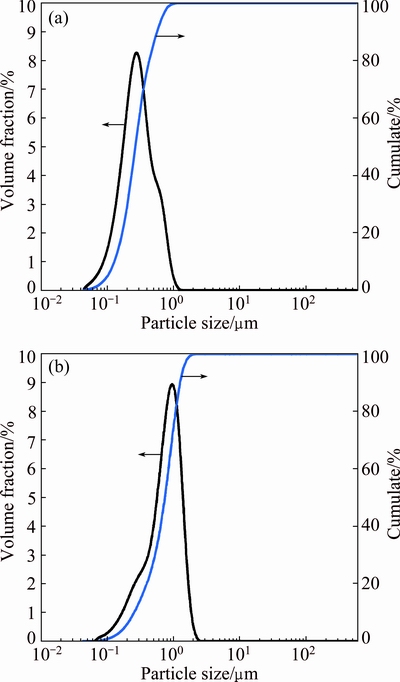
Fig. 2 Particle size distribution of starting 300 nm SnO2 powder (a) and 800 nm SnO2 powder (b)
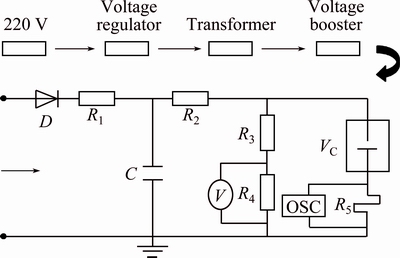
Fig. 3 Circuit of vacuum electrical breakdown
3 Results and discussion
3.1 Effect of SnO2 particle size on microstructure of Ag-4%SnO2 contact material
The morphologies and EDS mappings of Ag-SnO2 mixed powders prepared by 300 nm SnO2 powder and 800 nm SnO2 powder are given in Fig. 4. The red regions in Figs. 4(a2) and (b2) represent the distribution of Ag, and the green regions in Figs. 4(a3) and (b3) represent the distribution of Sn, while the yellow regions in Figs. 4(a4) and (b4) represent the distribution of O. As seen from Fig. 4, the Ag-SnO2 powders are mixed well after milling for 6 h at a mass ratio of ball to powder of 40:1 and a rotational speed of 200 r/min.
Figures 5(a) and (b) show the microstructures of Ag-4%SnO2 contact materials prepared by 300 and 800 nm SnO2 powders, respectively. The dark regions are SnO2 particles, while the gray regions are Ag matrix. As seen from Fig. 5(a), the distribution of SnO2 particles is relatively uniform on the Ag matrix for the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder. However, when the SnO2 particle size is 800 nm, there exists an obvious agglomeration in the Ag-4%SnO2 contact material, as shown in Fig. 5(b).
3.2 Effect of SnO2 particle size on properties of Ag- 4%SnO2 contact material
Table 1 lists the relative density, hardness and electrical conductivity of Ag-4%SnO2 contact materials prepared by 300 and 800 nm SnO2 powders. With the increase of SnO2 particle size, the relative density and hardness of Ag-4%SnO2 contact material decrease, while the electrical conductivity increases. This is because fine SnO2 particles increase the contact area between the Ag matrix and SnO2 particles, thus promoting the formation and growth of sintering necks. Moreover, fine SnO2 particles have larger surface energy, which is beneficial for sintering, giving rise to more densification of Ag-4%SnO2 contact material. The difference of hardness can be derived from the different strengthening effects of SnO2 particle sizes. According to the Hall-Petch equation [27], the hardness of AgSnO2 contact material increases with the decrease of SnO2 size. Compared with the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder, the electrical conductivity of Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder is increased by 62.60%. This can be ascribed to the combined effect of the poor densification and interfaces to electron scattering [28], but the interfaces may play a more significant role, thus giving rise to a higher electrical conductivity for the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder.
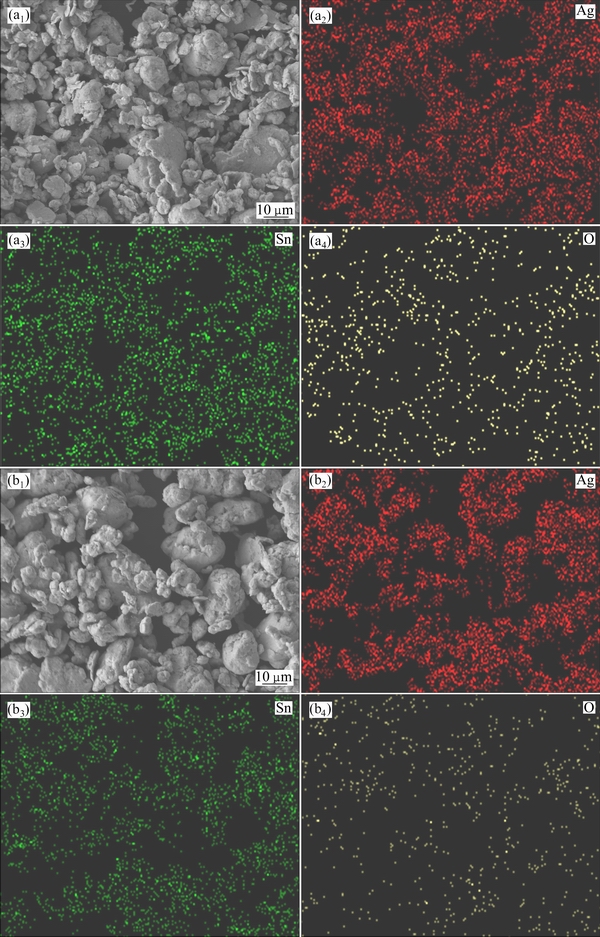
Fig. 4 Morphologies (a1, b1) and EDS mappings (a2-a4, b2-b4) of Ag-SnO2 mixed powders prepared with 300 nm SnO2 powder (a1-a4) and 800 nm SnO2 powder (b1-b4)
3.3 Effect of SnO2 particle size on arc erosion of Ag-4%SnO2 contact material
Figures 6(a) and (b) are the change of arc duration with operation times of Ag-4%SnO2 contact materials prepared with 300 and 800 nm SnO2 powders, respectively. It can be seen from Fig. 6(a) that the arc duration of Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder has a less fluctuation at the first 20 times and a large fluctuation at the last 30 times, and the average arc duration is 15.9 ms with a standard deviation of 1.93. However, for the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder, there is a large fluctuation of arc duration during the whole process, and the average arc duration is 19.27 ms with a standard deviation of 2.17, as shown in Fig. 6(b). The reason why the arc duration of Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder has a less change is that the fine SnO2 particles distribute uniformly in the Ag matrix. The surface of Ag-4%SnO2 contact material suffers less deterioration at the early stage of arc erosion, resulting in the approximate arc duration. With the increase of operation times, the accumulated arc energy leads to the formation of uneven surface, causing a larger fluctuation of arc duration. When SnO2 particles are coarse, the ion bombardment and splash of molten droplets are prone to occur, and the surface of Ag-4%SnO2 contact material deteriorates seriously, which leads to a large fluctuation of arc duration. This suggests that fine SnO2 particles are favorable for the improvement on the arc erosion resistance.
As for the better arc erosion resistance of Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder, it can be explained as follows. The fine SnO2 particles cannot offer enough metallic vapors to sustain arc combustion for a long time. Moreover, the SnO2 particles in the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder disperse in the Ag matrix more uniformly and the arc erosion becomes relatively dispersive when compared with those in the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder. The combined effect of the two factors gives rise to better arc erosion resistance of Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder.
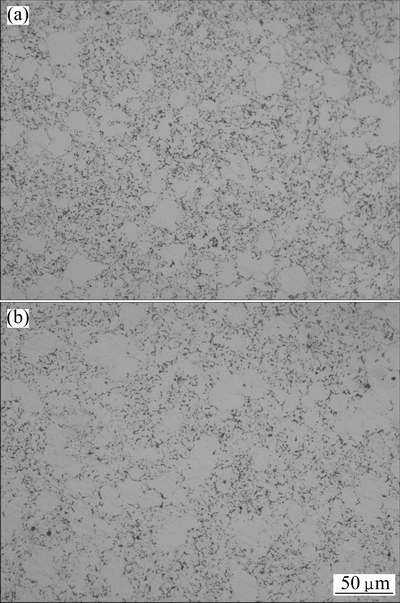
Fig. 5 Microstructures of Ag-4%SnO2 contact materials prepared by 300 nm SnO2 powder (a) and 800 nm SnO2 powder (b)
Table 1 Relative density, hardness and electrical conductivity of Ag-4%SnO2 contact materials prepared with different SnO2 particle sizes
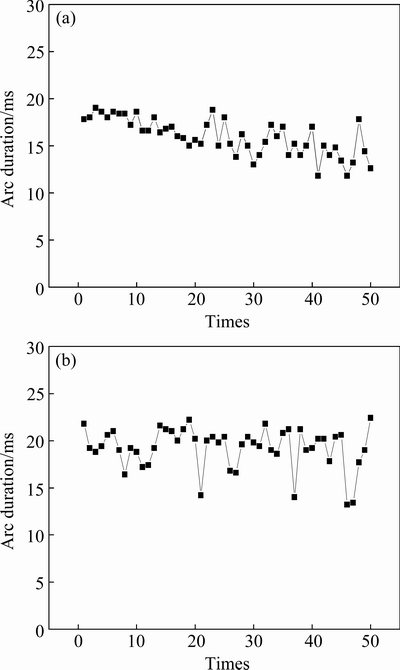
Fig. 6 Change of arc duration with operation times for Ag-4%SnO2 contact materials prepared with 300 nm SnO2 powder (a) and 800 nm SnO2 powder (b)
The mass of Ag-4%SnO2 contact materials prepared with 300 and 800 nm SnO2 powders before and after arc erosion 50 times are listed in Table 2. It is obvious that the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder has more mass loss than that prepared with 300 nm SnO2 powder. This is in good agreement with the arc duration.
Table 2 Mass change of Ag-4%SnO2 contact materials prepared with different SnO2 particle sizes before and after arc erosion 50 times
Figure 7 shows the surface morphologies of Ag-4%SnO2 contact materials prepared with 300 and 800 nm SnO2 powders after arc erosion 50 times at different magnifications. It can be seen from Figs. 7(a1) and (b1) that the arc erosion occurs on a larger area for the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powders than that for the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder, and the erosion areas measured are 4.56 and 3.23 mm2, respectively. As seen from Figs. 7(a2) and (a3), more dispersed and shallower erosion pits present on the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder. With the increase of SnO2 particle size, the arc erosion area becomes more concentrated, thus leading to the formation of larger and deeper erosion pits, as shown in Figs. 7(b2) and (b3). Additionally, it can also be seen from Fig. 7(b3) that there are obvious traces of flow and solidification of molten silver, spherical particles and large pits. This suggests that fine SnO2 particle size is beneficial for the improvement of the arc erosion resistance of Ag-4%SnO2 contact material.
Generally, it is believed that arc erosion prefers to occur on the phase with a low electron work function, and the arc motion is closely related to the phase size [29,30]. Since the electron work functions of Ag and SnO2 are 4.70 and 3.54 eV, respectively, the arc prefers to generate on the SnO2 particles. For coarse SnO2 particles, the arc can dwell on a site for a long period until it cannot be sustained, and subsequently hops to the adjacent one. The accumulated thermal energy results in larger and much deep erosion pits. However, the metallic vapor for arc to combustion is relatively less for fine SnO2 particles. Once SnO2 is exhausted, the arc moves to another site, resulting in a rapid arc motion. As a result, arc erosion is concentrated on a larger area and the erosion pits become shallower.
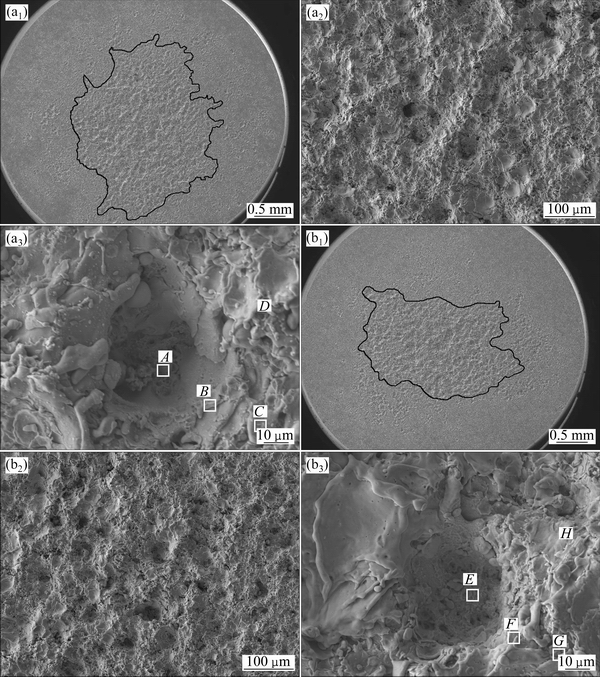
Fig. 7 Surface morphologies of Ag-4%SnO2 contact materials prepared with 300 nm SnO2 powder (a1-a3) and 800 nm SnO2 powder (b1-b3) after arc erosion 50 times
The effect of SnO2 particle size on the characteristics of arc motion can be further verified by the marginal surface morphologies of eroded Ag- 4%SnO2 contact materials prepared with 300 and 800 nm SnO2 powders, as shown in Figs. 8(a) and (b). As seen from Fig. 8(a), the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder has more continuous erosion pits, whereas the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder presents discontinuous erosion pits, as shown in Fig. 8(b). This demonstrates that the arc motion changes from random hopping to relatively continuous movement with the decrease of SnO2 particle size.
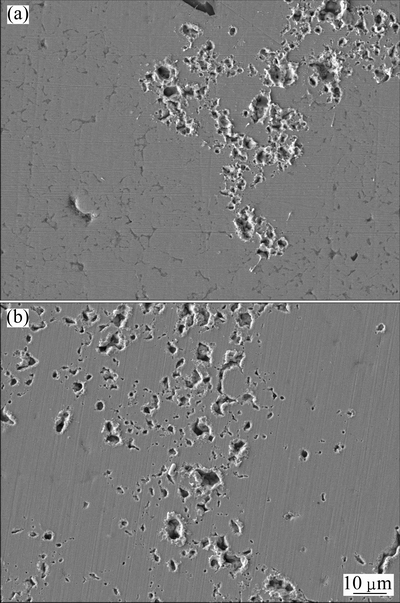
Fig. 8 Marginal surface morphologies of eroded Ag-4%SnO2 contact materials prepared with 300 nm SnO2 powder (a) and 800 nm SnO2 powder (b) after arc erosion 50 times
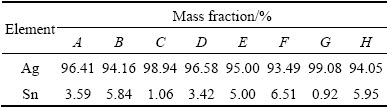
To determine the surface compositions of eroded Ag-4%SnO2 contact materials, point analyses by EDS in zones A, B, C, D, E, F, G and H shown in Fig. 7 are listed in Table 3. From the EDS analyses of points A, B, C, E, F and G, it is clear that the surface composition of Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder is relatively homogeneous. The EDS results of zones D and H show that the latter has a higher SnO2 content, which indicates that more pronounced erosion of the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder occurs in comparison with that of the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder. This is derived from the poor wettability between molten Ag and SnO2 particles. According to the analysis of acting force to the SnO2 particles (Eq. (1)), the floating acceleration of SnO2 particles in the molten pool is achieved, as shown in Eq. (2). It is learnt from Eq. (2) that the large SnO2 particles are more easier to reach the surface, which leads to the decreased viscosity at the bottom of the molten pool, so the splash of the molten Ag occurs easily. Whereas, fine SnO2 particles can dwell on the molten pool for a long time, thus inhibiting the sputter erosion under the turbulent of molten pool. Consequently, the Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder exhibits better arc erosion resistance.
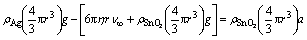 (1)
(1)
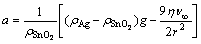 (2)
(2)
where ρSnO2 and ρAg are the densities of SnO2 and Ag, respectively, r is the radius of SnO2 particle, η and ν∞ are the coefficient of dynamic viscosity and kinematic viscosity, respectively, and g is the acceleration of gravity.
Table 3 EDS results of zones A, B, C, D, E, F, G and H shown in Fig. 7
4 Conclusions
1) Fine SnO2 particles increase the relative density and hardness of Ag-4%SnO2 contact material, while decrease the electrical conductivity.
2) The Ag-4%SnO2 contact material prepared with 300 nm SnO2 powder has shorter arc duration and less mass loss in comparison with the Ag-4%SnO2 contact material prepared with 800 nm SnO2 powder.
3) Fine SnO2 particles enhance the arc erosion resistance of Ag-4%SnO2 contact material. The Ag-4%SnO2 contact material prepared with 300 nm SnO2 powders presents larger erosion area and shallower and more dispersed arc erosion pits.
References
[1] WANG X H, YANG H, CHEN M, ZOU J T, LIANG S H. Fabrication and arc erosion behaviors of AgTiB2 contact materials [J]. Powder Technology, 2014, 256: 20-24.
[2] TAO Qi-ying, ZHOU Xiao-long, ZHOU Yun-hong, ZHANG Hao. Electrical contact properties of AgCuO electrical contact materials [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(5): 1244-1249. (in Chinese)
[3] COSOVIC V, COSOVIC A, TALIJAN N, ZIVKOVIC D, MANASIJEVIC D, MINIC D. Improving dispersion of SnO2 nanoparticles in Ag-SnO2 electrical contact materials using template method [J]. Journal of Alloys and Compounds, 2013, 567: 33-39.
[4] WU C P, YI D Q, LI J, XIAO L R, WANG B, ZHENG F. Investigation on microstructure and performance of Ag/ZnO contact material [J]. Journal of Alloys and Compounds, 2008, 457: 565-570.
[5] YE Jia-jian, XIONG Wei-hao, XU Jian, FU Jiang-hua, LI Zhen-biao. Research progress in AgSnO2 contact material in low-voltage relay [J]. Materials Review, 2007, 21(2): 87-90. (in Chinese)
[6] BIYIK S, ARSLAN F, AYDIN M. Arc-erosion behavior of boric oxide-reinforced silver-based electrical contact materials produced by mechanical alloying [J]. Journal of Electronic Materials, 2015, 44(1): 457-466.
[7] WANG J, LI D M, WANG Y P. Microstructure and properties of Ag-SnO2 materials with high SnO2 content [J]. Journal of Alloys and Compounds, 2014, 582: 1-5.
[8] COSOVIC V, PAVLOVIC M, COSOVIC A, VULIC P, PREMOVIC M, ZIVKOVIC D, TALIJAN N. Microstructure refinement and physical properties of Ag-SnO2 based contact materials prepared by high-energy ball milling [J]. Science of Sintering, 2013, 45(2): 173-180.
[9] ZHOU Zhao-feng, GAN Wei-ping. Development of AgSnO2 contact material [J]. Rare Metals and Cemented Carbides, 2004, 32(2): 53-56. (in Chinese)
[10] LIN Z J, LIU S H, SUN X D, XIE M, LI J G, LI X D, CHEN Y T, CHEN J L, HUO D, ZHANG M, ZHU Q, LIU M M. The effects of citric acid on the synthesis and performance of silver-tin oxide electrical contact materials [J]. Journal of Alloys and Compounds, 2014, 588: 30-35.
[11] XU Can-hui, YI Dan-qing, CAO Shi-yi, LIU Hui-qun, WU Chun-ping, SUN Shun-ping, LIU Run-yong. Hot compression behavior of AgSnO2 composite material [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2091-2098. (in Chinese)
[12] YANG Tian-zu, DU Zuo-juan, GU Ying-ying, QIU Xiao-yong, JIANG Ming-xi, CHU Guang. Preparation of flake AgSnO2 composite powders by hydrothermal method [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(2): 434-438.
[13] RIEDER W, WEICHSLER V. Make erosion mechanism of AgCdO and AgSnO2 contact [J]. IEEE Transactions on Components, Hybrids, and Manufacturing Technology, 1992, 15(3): 332-338.
[14] RONG Ming-zhe. Theory of electrical contact [M]. 1st ed. Beijing: China Machine Press, 2004: 27-29. (in Chinese)
[15] BEHRENS V, HONIG T, KRAUS A, MICHAL R, SAEGER K, SCHMIDBERGER R, STANEFF T. An advanced silver/tin oxide contact material [C]//Proceeding of the 39th IEEE Holm Conference on Electrical Contacts. Pittsburgh: IEEE, 1993: 19-25.
[16] DU Yong-guo, YANG Guang, ZHANG Jia-chun, WANG Guang-hua, LONG Yan. Physical metallurgy analysis of arc erosion of AgMeO contact material [J]. Electrical Engineering Materials, 1997, 4: 1-8. (in Chinese)
[17] ZHU Yan-cai, WANG Jing-qin, WANG Hai-tao. Study on arc erosion resistance properties of nano-AgSnO2 electrical contact materials doped with Bi [J]. Rare Metal Material and Engineering, 2013, 42(1): 149-152. (in Chinese)
[18] ZHU Y C, WANG J Q, WANG H T, DING J. Preparation and study on nano-Ag/SnO2 electrical contact material doped rare earth element [J]. Materials Science Forum, 2014, 789: 270-274.
[19] LUNGU M, GAVRILIU S, CANTA T, LUCACI M, ENESCU E. AgSnO2 sintered electrical contacts with ultrafine and uniformly dispersed microstructure [J]. Journal of Optoelectronics and Advanced Materials, 2006, 8(2): 576-581.
[20] WANG Jun-bo, LI Ying-min, WANG Ya-ping, DING Bing-jun. Study of nanocomposite silver-based contact materials [J]. Rare Metal Material and Engineering, 2004, 33(11): 1213-1217. (in Chinese)
[21] WITTER G, CHEN Z. A comparison of silver tin indium oxide contact materials using a new model switch that simulates operation of an automotive relay [C]//Proceeding of the 50th IEEE Holm Conference on Electrical Contact and the 22nd International Conference on Electrical Contacts. Seattle: IEEE, 2004: 382-387.
[22] SWINGLER J, SUMPTION A. Arc erosion of AgSnO2 electrical contacts at different stages of a break operation [J]. Rare Metals, 2010, 29(3): 248-254.
[23] XU Jian, XIONG Wei-hao, FU Jiang-hua, LI Zhen-biao. Simulation analysis of effect of second metal-oxide phase on molten pool in Ag-based electrical contact [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(7): 1323-1329. (in Chinese)
[24] ZHANG Kun-hua, GUAN Wei-ming, SUN Jia-lin, LU Feng, CHEN Jing-chao, ZHOU Xiao-long, DU Yan. Preparation and DC arc erosion morphology of AgSnO2 contact materials [J]. Rare Metal Material and Engineering, 2005, 34(6): 924-927. (in Chinese)
[25] LIN Z J, SUN X D, LIU S H, CHEN J L, XIE M, LI J G, LI X D, HUO D, ZHANG M, ZHU Q. Effect of SnO2 particle size on properties of Ag-SnO2 electrical contact materials prepared by the reductive precipitation method [J]. Advanced Materials Research, 2014, 936: 459-463.
[26] BRAUMANN P, KOFFLER A. The influence of manufacturing process, metal oxide content, and additives on the switching behavior of Ag/SnO2 in relays [C]//Proceeding of the 50th IEEE Holm Conference on Electrical Contact and the 22nd International Conference on Electrical Contacts. Seattle: IEEE, 2004: 90-97.
[27] HANSEN N. Hall—Petch relation and boundary strengthening [J]. Scripta Materialia, 2004, 51(8): 801-806.
[28] DU Yong-guo, BAI Shu-xin, YI Yang, ZHANG Jia-chuan. The resistance of tin-oxide particulate-reinforced silver composite materials [J]. Journal of Functional Materials, 1994, 25(2): 150-153. (in Chinese)
[29] WANG Ya-ping, ZHANG Li-na, DING Bing-jun, ZHOU Jing-en. Effect of selective strengthening of CuCr contact materials on the dielectric strength in a short vacuum gap [J]. Proceedings of the Chinese Society for Electrical Engineering, 1999, 19(3): 47-50. (in Chinese)
[30] WANG Ya-ping, ZHANG Hui, DING Bing-jun, SUN Jun. Influence of the microstructure of electrode materials on the motion behaviors of vacuum arc cathode spot [J]. Acta Metallurgica Sinica, 2004, 40(12): 1269-1273. (in Chinese).
张 苗,王献辉,杨晓红,邹军涛,梁淑华
西安理工大学 陕西省电工材料与熔(浸)渗技术重点实验室,西安 710048
摘 要:为了阐明SnO2粒度大小对AgSnO2触头材料电弧侵蚀行为的影响,采用粉末冶金法制备不同SnO2粒度的Ag-4%SnO2(质量分数)触头材料,对触头材料组织进行观察,并对其致密度、硬度和导电率进行测量。对Ag-4%SnO2触头材料进行电弧侵蚀实验,确定燃弧时间和电弧侵蚀前后的质量变化,并对电弧侵蚀后触头材料表面的形貌和成分进行表征。结果表明:细小的SnO2颗粒有助于提高Ag-4%SnO2触头材料的致密度和硬度,但降低了其导电率。随着SnO2粒度的减小,Ag-4%AgSnO2触头材料的燃弧时间变短,质量损失降低,电弧侵蚀面积增大,蚀坑变浅且分散。
关键词:AgSnO2触头材料;SnO2粒度;电弧侵蚀;导电率;硬度
(Edited by Mu-lan QIN)
Foundation item: Project (51274163) supported by the National Natural Science Foundation of China; Project (13JS076) supported by the Key Laboratory Research Program of Shaanxi Province, China; Project (2012KCT-25) supported by the Pivot Innovation Team of Shaanxi Electrical Materials and Infiltration Technique, China; Project (2011HBSZS009) supported by the Special Foundation of Key Disciplines, China
Corresponding author: Xian-hui WANG; Tel: +86-29-82312185; E-mail: xhwang693@xaut.edu.cn
DOI: 10.1016/S1003-6326(16)64168-7

