Article ID: 1003-6326(2005)06-1351-05
Structure and electrochemical properties of La-Mg-Ni system hydrogen storage alloys with different Co contents
WANG Jian-hui(王建辉), ZHONG Kai(钟 凯), DING Hui(丁 慧),
LI Rui(李 锐), GAO Ming-xia(高明霞), PAN Hong-ge(潘洪革)
(Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China)
Abstract: The structure and electrochemical properties of the La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) hydrogen storage alloys were investigated. The crystal structure and the lattice parameters of the alloys were analyzed by X-ray diffractometry and Rietveld method. Electrochemical properties of the alloys including p—c—t curves, discharge capacity, discharge capacity retention were studied. The results show that (La, Mg)Ni3 and LaNi5 are the main phases of all the alloys. The plateau pressure for hydrogen absorption/desorption decreases and the hydrogen storage capacity firstly increases and subsequently decreases with increasing Co content. The values of the maximum discharge capacity of the alloy electrodes remain in range of 395.3-403.1mA·h/g in spite of the change of Co content. The cycling stability of the alloy electrodes is greatly improved with increasing Co content, which is attributed to the suppression of the cell volume expansion during hydriding, leading the pulverization of the alloy particles lowered and the oxidation/corrosion of the active elements reduced.
Key words: La-Mg-Ni alloy; Ni/MH batteries; structure; hydrogen storage alloys; p—c—t curve; electrochemical properties CLC
number: TG139.7 Document code: A
1 INTRODUCTION
To increase the discharge capacity of nickel/metal-hydride(Ni/MH) batteries, new types of hydrogen storage alloys with higher energy density have been paid considerable attention by researchers. Particularly, recent investigations on the R-Mg-Ni (R= rare earth or Ca element) system hydrogen storage alloys have led to a new series of ternary alloys with a high hydrogen storage capacity[1-3]. Kohno et al[4] found that the maximum discharge capacity of the La0.7Mg0.3Ni2.8Co0.5 alloy electrode could reach 410mA·h/g during the researches of the La2MgNi9, La5Mg2Ni23 and La3MgNi14 alloy systems. Moreover, Liu et al[5-8] also revealed that suitable heat treatment and partial substitution of Mn for Ni could improve the overall properties of the La-Mg-Ni-Co type electrode alloys, and the discharge capacity of 380-410mA·h/g was reached. However, the cycling stability of this kind of alloys is still poor for commercial applications[8-11], further investigations on the improvement of the cycling durability of the La-Mg-Ni-Co hydrogen storage alloys are highly necessary. It is known that the appearance of Co can effectively improve the cycling stability of the rare earth-based alloys due to the decrease of the pulverization of the alloy particles and the corrosion of the alloy electrodes in alkaline solution[12-15].
In this paper, at the aim of improving the cyclic lifetime, Co was selected to partially substitute Ni and the structure, hydrogen storage and electrochemical properties of the La0.7Mg0.3Ni3.4-x-Mn0.1Cox (x=0, 0.3, 0.6, 0.75, 0.9, 1.05) hydrogen storage alloys have been investigated systematically.
2 EXPERIMENTAL
La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) alloys were prepared by induction levitation melting in a water-cooled copper crucible under argon atmosphere. The purity of the metal components is higher than 99.5%(mass fraction). The ingots were turned over and remelted twice for homogeneity. Then the ingots were crushed and mechanically milled into powder of size of 30-80μm for pressure-composition isotherm (p—c—t) and electrochemical measurements and X-ray diffraction(XRD) analyses of the crystal structure.
The X-ray data were obtained with an ARL X-ray diffractometer using CuKα radiation. Pressure-composition isotherms (p—c—t) for hydrogen absorption/desorption were measured by the Sieverts method using an automatic Sieverts-type apparatus at 303K.
The alloy electrodes were prepared by mixing 0.1g alloy powders with 0.4g carbonyl nickel powder and then the mixture was cold pressed into a pellet up to pressure of 16MPa. Electrochemical studies were carried out in a half-cell consisting of a working electrode (the MH electrode for studying), a sintered Ni(OH)2/NiOOH counter electrode and a Hg/HgO reference electrode. The electrolyte was 6mol/L KOH solution. Each electrode was charged at 100mA/g for 5h followed by a 10min rest and then discharged at 60mA/g to the cut-off potential of -0.6V (vs Hg/HgO).
3 RESULTS AND DISCUSSION
Fig.1 shows the XRD patterns of the La0.7-Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) hydrogen storage alloys. Fig.2 shows the example of XRD patterns and Rietveld analysis patterns of the alloy with x=0.75. It can be seen from Fig.1 that (La, Mg)Ni3 and LaNi5 are the main phases of all of the alloys, additionally, minor phase of LaNi was also detected.
Table 1 lists the lattice parameters and cell volumes of the La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0- 1.05) alloys, which was calculated from the data of Fig.1 by using Rietveld method. Data in Table 1 show that parameters of a and c of both(La, Mg)Ni3 phase and LaNi5 phase increase with
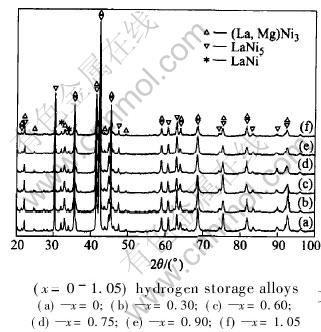
Fig.1 XRD patterns of La0.7Mg0.3Ni3.4-xMn0.1Cox
Table 1 Characteristics of alloy phases in La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0 -1.05) hydrogen storage alloys
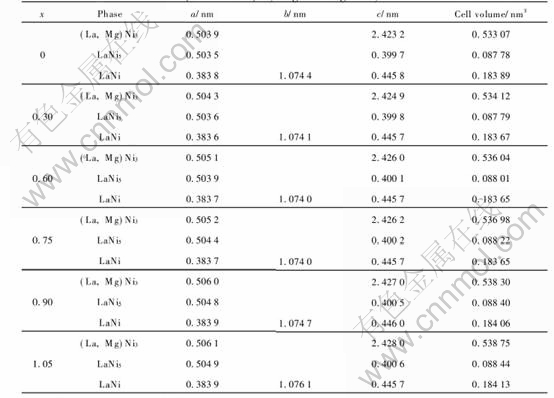
Fig.2 XRD patterns and Rietveld analysis patterns of La0.7Mg0.3Ni3.4-xMn0.1Cox alloy with x=0.75
increasing Co content, leading a subsequent expansion of the cell volumes, which was caused by the larger atomic radius of Co (0.167nm) compared with Ni (0.162nm).
Fig.3 shows the pressure-composition isotherms (p—c—t) of La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) at 303K. With increasing value of x from 0 to 1.05, the equilibrium pressure for hydrogen absorption/desorption of these alloys decreases gradually, which can be attributed to that the cell volumes are enlarged after the partial substitution of Co for Ni. In addition, the maximum hydrogen storage capacity of the alloys firstly increases from 1.44% (x=0) to 1.55% (x=0.75) and then decreases to 1.48% (x=1.05), which is related to the change of the content of the (La, Mg)Ni3 phase and the LaNi5 phase in the alloys and the decrease of the plateau pressure for hydrogen absorption/desorption.
Fig.4 shows the curves of discharge capacity vs cycle times of the La0.7Mg0.3Ni3.4-xMn0.1Cox(x=0-1.05) alloy electrodes at 303K. It can be seen that the maximum discharge capacities almost remain stable when the Co content increases from x=0 to x=1.05, just increase a little from 397.5(x=0) to 403.1mA·h/g (x=0.75) then decrease to 395.3mA·h/g (x=1.05). This is in good agreement with the result obtained from the p—c—t measurements and is also attributed to the reducing of the plateau pressure of hydrogen absorption/desorption and the different content of (La, Mg)Ni3 phase and LaNi5 phase. Fig.4 further reveals that all of the alloy electrodes can be easily activated to their maximum discharge capacity within two cycles. Moreover, the cycling stability is greatly improved during the cycling.
Fig.5 displays the discharge capacity retention
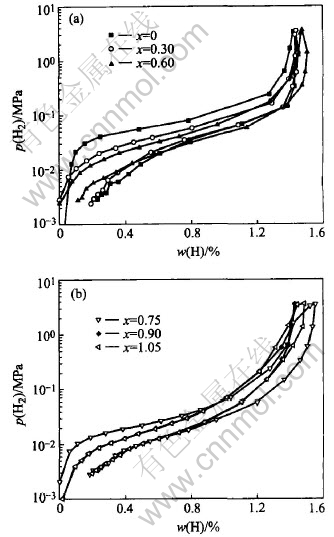
Fig.3 p—c—t isotherms curves of La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) hydrogen storage alloys at 303K
of the La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) alloy electrodes after 90 charge/discharge cycles. The discharge capacity retention of the alloy electrodes increases obviously from 25.5% to 47.9% with Co content increasing from x=0 to x=1.05. This is mainly attributed to the lowering of the cell volume expansion (ΔV/V) and a reducing of the oxidation/corrosion of the active elements in the alloys[12-15].
Fig.6 shows the XRD patterns of the La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0, 0.6, 0.75, 0.9) alloys after be fully charged. It can be seen that the hydride phases of the (La, Mg)Ni3 and LaNi5 remain the original PuNi3-type structure and CaCu5-type structure, respectively. Moreover, the diffraction peaks of hydride phases shift toward larger angles with increasing Co content, indicating that the cell volume expansion caused by hydrogenation reduces.
Table 2 lists the lattice parameters and unit cell volumes of the (La, Mg)Ni3 phase and LaNi5 phase after charging, which was calculated from the data of Fig.6 by using Rietveld method. It is
Table 2 Characteristics of hydride phases in La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) hydrogen storage alloys
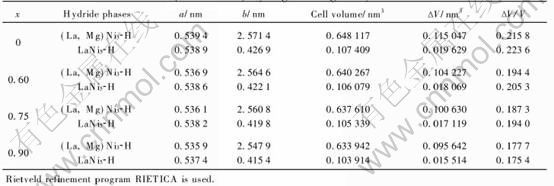
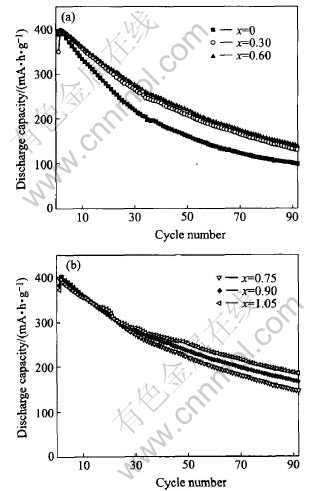
Fig.4 Discharge capacity vs cycle numbers of La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) alloy electrodes at 303K
obvious that the volume expansion ratio (ΔV/V) of the (La, Mg)Ni3-H phase decreases from 21.58% to 17.76% when the Co content increasing from x=0 to x=0.9, and that of the LaNi5-H phase decreases from 22.36% to 17.54%. This reveals that the alloy electrodes undergo a smaller cell volume expansion and contraction in each charge/discharge cycle with increasing Co content, and lower pulverization of the alloy particles. Thus, the oxida- tion/corrosion of the active elements in the alloys
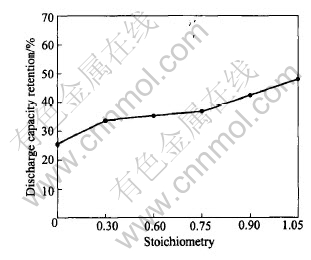
Fig.5 Discharge capacity retention of La0.7Mg0.3NiM3.4-xMn0.1 (x=0-0.15) alloy electrode after 90 charge/discharge cycles
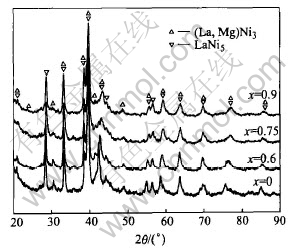
Fig.6 XRD patterns of La0.7Mg0.3Ni3.4-xCox (x=0, 0.60, 0.75, 0.90) hydrides
is reduced. The improvement of the cycling durability of the alloy electrodes with increasing Co content can also be obviously seen from Fig.4.
4 CONCLUSIONS
(La, Mg)Ni3 and LaNi5 phase are the main phases of La0.7Mg0.3Ni3.4-xMn0.1Cox (x=0-1.05) hydrogen storage alloys, additionally, small amount of the LaNi phase was also existed. The lattice parameters and cell volumes of the (La, Mg)-Ni3 phase and the LaNi5 phase all increase with increasing Co content. The equilibrium pressure for hydrogen decreases gradually with increasing Co content. The maximum electrochemical discharge capacity is almost in the same value, being of about 400mA·h/g when the Co content varies from x= 0 to x=1.05. But the cyclic stability of the alloy electrodes was significantly improved with increasing Co content, which was attributed to the suppression of the cell volume expansion during hydriding, leading the pulverization of the alloy particles lowered and thus the oxidation/corrosion of the active elements reduced.
REFERENCES
[1]Kadir K, Sakai T, Uehara I. Synthesis and structure determination of a new series of hydrogen storage alloys; RMg2Ni9 (R=La, Ce, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type alternating with AB5 layers [J]. J Alloys Compd, 1997, 257(1-2): 115-121.
[2]Kadir K, Sakai T, Uehara I. Structural investigation and hydrogen capacity of LaMg2Ni9 and (La0.65Ca0.35)-(Mg1.32Ca0.68)Ni9 of the AB2C9 type structure [J]. J Alloys Compd, 2000, 302(1-2): 112-117.
[3]Latroche M, Percheron-Guégan A. Structural and thermodynamic studies of some hydride forming RM3-type compound (R=Lanthanide, M=transition metal) [J]. J Alloy Compd, 2003, 356-357: 461-468.
[4]Kohno T, Yoshida H, Kawashima F, et al. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14 [J]. J Alloys Compd, 2000, 311(2): L5-L7.
[5]Liu Y F, Pan H G, Gao M X, et al. Hydrogen storage and electrochemical properties of the La0.7Mg0.3-Ni3.825-xCo0.675Mnx hydrogen storage electrode alloys [J]. J Alloys and Comp, 2004, 365 (1-2): 246-252.
[6]Liu Y F, Pan H G, Gao M X, et al. Influences of heat treatment on electrochemical characteristics of La0.75-Mg0.25Ni2.8Co0.5 hydrogen storage electrode alloy [J]. Trans Nonferrous Met Soc China, 2003, 13: 25-28.
[7]Liu Y F, Pan H G, Gao M X, et al. Influence of Mn content on the structural and electrochemical properties of the La0.7Mg0.3Ni4.25-xCo0.75Mnx hydrogen storage alloys [J]. Mater Sci Eng A, 2004, A372(1-2): 163-172.
[8]Pan H G, Liu Y F, Gao M X, et al. The structural and electrochemical properties of the La0.7Mg0.3-Ni2.975-xCo0.525Mnx hydrogen storage electrode alloys [J]. J Electrochem Soc, 2004, 151(3): A 374-A380.
[9]Addour-Hadjean R B, Meyer L, Pereira-Ramos J P, et al. An electrochemical study of new La1-xCexY2Ni9 (0≤x≤1) hydrogen storage alloys [J]. Electochem Acta, 2001, 46: 2385-2393.
[10]Chen J, Kuriyama N, Takeshita H T, et al. Hydrogen storage alloys with PuNi3-type structure as metal hydride electrodes [J]. Electrochem Solid-State Lett, 2000, 3(6): 249-252.
[11]LIU Li-qin, TANG Rui, LIU Yong-ning. Effects of rare earth elements on properties of La0.8Mg0.2Ni2.8-Co0.6 hydrogen storage alloy [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 871-875.(in Chinese)
[12]Willems J J G. Metal hydride electrodes stability of LaNi5-related compounds [J]. Philips J Res, 1984, 39(S1): 1-94.
[13]Sakai T, Oguro K, Miyamura H, et al. Some factors affecting the cycle lives of LaNi5-based alloy electrodes of hydrogen batteries [J]. J Less-Common Met, 1990, 161: 193-202.
[14]Pan H G, Liu Y F, Gao M X, et al. A study of the structural and electrochemical properties of La0.7-Mg0.3(Ni0.85Co0.15)x (x=2.5-5.0) hydrogen storage electrode alloys [J]. J Electrochem Soc, 2003, 150: A565-A570.
[15]Vogt T, Reilly J J, Johnson J R, et al. Site preference of cobalt and deuterium in the structure of a complex AB5 alloy electrode [J]. J Electrochem Soc, 1999, 146: 15-19.
(Edited by LONG Huai-zhong)
Foundation item: Project(50131040) supported by the National Natural Science Foundation of China
Received date: 2005-03-25; Accepted date: 2005-08-15
Correspondence: PAN Hong-ge, Professor, PhD; Tel/Fax: +86-571-87952576; E-mail: honggepan@zjuem.zju.edu.cn