
Adsorption of sodium polyacrylate at interface of
dicalcium silicate-sodium aluminate solution
YU Hai-yan, PAN Xiao-lin, DING Ting-ting, ZHANG Wu, LIU Han, BI Shi-wen
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 30 October 2010; accepted 27 May 2011
Abstract: The adsorption isotherm of sodium polyacrylate on dicalcium silicate (2CaO·SiO2) in sodium aluminate solution at 80 °C was studied. The type of surface adsorption of sodium polyacrylate is saturated adsorption, and the adsorption behavior belongs to L-type, according with the monolayer adsorption model of Langmuir equation. The surface coverage of sodium polyacrylate is 1.06 mol/μm2. The relation curve between the surface pressure and the molecular area of adsorption film was obtained by Gibbs formula. The variation of interfacial energy caused by adsorption as well as the relationship between the relation curve and the type of adsorption was discussed.
Key words: sodium aluminate solution; sodium polyacrylate; dicalcium silicate; interface; adsorption
1 Introduction
In the sintering process of alumina production, about 30% of clinker is dicalcium silicate (2CaO·SiO2). During the leaching of clinker, as dicalcium silicate reacts with sodium aluminate solution, both Al2O3 and Na2O re-enter into the red mud, resulting in the loss of Al2O3 and Na2O [1]. It was found [2] that additions of surfactants, such as sodium polyacrylate and polyethylene glycol, can inhibit the secondary reaction of dicalcium silicate with sodium aluminate solution, which increases the leaching rate of Al2O3.
Polyacrylic acid and its salt sodium polyacrylate are the most frequently applied additives in the alumina industry [3-4]. Much work was done on the additives for inhibiting the secondary reaction, which was mainly concerned on the selection and application of additives as well as the adsorption kinetics and thermodynamics [5-7]. However, the basic theories, such as the models of adsorption, the laws at the interface of sodium aluminate solution and dicalcium silicate, and the effect of adsorption on the solid–liquid interface were seldom studied [8]. Consequently, according to the development of Gibbs adsorption isothermal formula at the solid-liquid interface [9-12], the adsorption laws of sodium polyacrylate in clinker–sodium aluminum solution systems and the mechanisms of sodium polyacrylate inhibiting the secondary reaction were studied.
2 Experimental
Dicalcium silicate was prepared by pure calcium oxide and silica. Calcium oxide and silica were first mixed with the molar ratio of 2 to 1, ground for 2 h in a mortar, and then calcined at 1350 ℃ followed by quenching in the air.
Sodium aluminate solution with caustic ratio (i.e. molar ratio of Na2O to Al2O3) of 1.30 was prepared by the red mud lotion from the Shanxi Branch of the China Aluminum Company, industrial sodium hydroxide and aluminum hydroxide, and analytically pure sodium carbonate. The concentrations of Na2O and Na2CO3 of sodium aluminate solution were 36 g/L and 15 g/L, respectively.
Sodium polyacrylate was added to sodium aluminate solution with a dicalcium silicate concentration of 75 g/L. The concentrations of sodium polyacrylate in sodium aluminate solution were 0, 80, 120, 160, 200, 240 mg/L, respectively. The mixed solutions were stirred magnetically (400 r/min) for 2 h in a 80 °C water bath followed by centrifugal separation. 0.5 mL supernatant fluid was taken out to determine the content of sodium polyacrylate by the method of liquid chromatography [13].
The adsorbed amount per unit mass of adsorbent at equilibrium, Qe, can be expressed as follows:
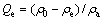 (1)
(1)
where ρ0 and ρe are the initial concentration and the residual concentration of additives (mg/L) in the solution, respectively; and ρa is the concentration of adsorbent (g/L) in the solution.
3 Gibbs adsorption isotheral formula
According to the interface Gibbs formula in the ideal solution [14], the basic form of surface tension, γ, under constant temperature and pressure is given by:
 (2)
(2)
where ci is the molar concentration of component i; ni is the surface molar quantity of component i; As is the surface area; μi is the chemical potential of component i; Γi is the excess amount of component i per unit surface (i.e. the surface adsorbed amount or surface concentration (mol/m2)).
As the interfacial tension numerically equals the interfacial pressure (π), the relationship is dπ =-dγ. Thus,
 (3)
(3)
For binary systems, if Group 1 is identified as the major component, Γ1(1)=0; the adsorbed amount of Group 2 is Γ2(1); then, Eq. (3) can be written as:
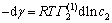 (4)
(4)
For the adsorption in the dilute solution, it is reasonable to consider the surface excess amount Γ2(1) as the actually measured amount (apparent adsorbed amount), so Γ2(1) is replaced by Γ and c2 is replaced by c in Eq. (3). For the solid adsorption from liquid, Eq. (3) is integrated on both sides:
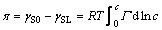 (5)
(5)
where γS0 and γSL are the solid–liquid interface free energy before and after adsorbing the solute, respectively.
The value of  with different concentrations can be obtained by graphic integration, and then the value of π with different adsorbed amounts can be calculated. At very low concentration, the relationship between Γ and ln c is considered a line.
with different concentrations can be obtained by graphic integration, and then the value of π with different adsorbed amounts can be calculated. At very low concentration, the relationship between Γ and ln c is considered a line.
Accordingly, the area of each adsorbed molecule (σ) at different amounts of Γ is given by the Avogadro constant (NA) as
 (6)
(6)
4 Results and discussion
4.1 Adsorption isotherm
The adsorption isotherm of sodium polyacrylate is shown in Fig. 1. The type of adsorption isotherm belongs to “Langmuir” type [15], which indicates that the adsorption of sodium polyacrylate on 2CaO·SiO2 accords with the Langmuir monolayer model, and follows the Langmuir equation expressed in Eq. (7):
 (7)
(7)
where Qm is the limited amount of adsorption, and b is a constant.
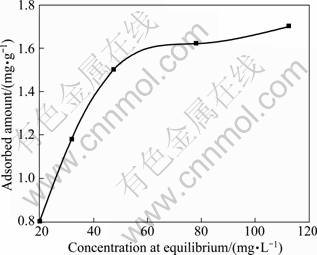
Fig. 1 Adsorption isotherm of sodium polyacrylate on dicalcium silicate at 80 °C
The relationship between the residual concentration (ρe) and the value of ρe/Qe is shown in Fig. 2. The curve can be fitted by the Langmuir equation as
 (8)
(8)
As shown in Fig. 2, the experimental data can be well simulated by the adsorption isotherm. The adsorption of sodium polyacrylate at the interface of dicalcium silicate-sodium aluminum solution belongs to “L”-type, having the characteristic of monolayer adsorption. The limited amount of adsorption (Qm) is calculated to be 2.17 mg/g.
As a nonionic polymer surfactant, the type of adsorption isotherm of polyethylene glycol on 2CaO·SiO2 in sodium aluminate solution belongs to “S”-type, and the adsorption of polyethylene glycol on 2CaO·SiO2 accords with the Freundlich multi-layer model [8], which indicates that the adsorption mechanisms of sodium polyacrylate and polyethylene glycol on 2CaO·SiO2 in sodium aluminate solution are different.

Fig. 2 Curve of ρe vs ρe/Qe
4.2 Adsorption film on dicalcium silicate
The π—σ curve of the interfacial film on dicalcium silicate can be obtained from the adsorption results by using Eqs. (5) and (6). As shown in Fig. 3, the value of π decreases with the increase of σ. As the adsorbed amount of sodium polyacrylate on dicalcium silicate increases, both the solid surface energy and the interfacial tension decrease, indicating that the adsorption of sodium polyacrylate occurs spontaneously. Meanwhile, the variation of π—σ curve at the solid-liquid interface can clearly show the existing state of the adsorption film, that is, the form of adsorption film on dicalcium silicate is a single liquid-expanded film. The results about the adsorption film are in accordance with the “L”-type adsorption isotherm of sodium polyacrylate, which further confirms the monolayer adsorption at the interface. The saturation coverage of sodium polyacrylate on dicalcium silicate is calculated to be 1.06 mol/?μm2.
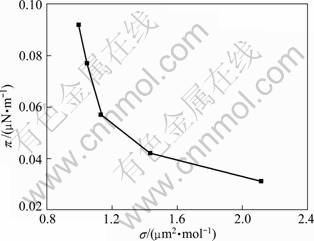
Fig. 3 Relationship between interfacial pressure and molecular area
It is well known that sodium polyacrylate is an anionic polymer surfactant with the structural formula [ CH2—CH(COONa) ]n . As the molecular chain of sodium polyacrylate is very long, multi-points and various forces exist. When the concentration of sodium polyacrylate is very low, electrostatic forces on the surface of dicalcium silicate are formed by —COOH in the active ions of polymer, and hydrogen bonds are also formed by the hydroxyl (—OH) and the surface bonding hydroxyl (—OH). As a result, the molecules of sodium polyacrylate are adsorbed over the surface of dicalcium silicate with no rules, so that each molecule has a comparatively large area. When the concentration of sodium polyacrylate tends to saturation, almost all of the reactive groups on the adsorbent surface involve in the reaction, and the interfacial tension reaches the minimum value. If π equals zero in the π-σ curve, the corresponding cross-sectional area is calculated to be about 1.60 μm2, which is in consistent with the orientation of molecules to stand or to lie on the surface. Therefore, the saturated monolayer structure of sodium polyacrylate may not be in a vertical orientation.
5 Conclusions
1) The adsorption of sodium polyacrylate at the interface of dicalcium silicate-sodium aluminate solution is a saturated monolayer adsorption and the type of adsorption is “L”-type, in accordance with the Langmuir equation.
2) As the hydrogen bonds are the main forces for the adsorption of sodium polyacrylate on the surface of dicalcium silicate, the adsorption belongs to chemical adsorption. The π-σ curve in sodium aluminate solution can be drawn according to the Gibbs formula, which is available to obtain useful information from the shape of the π-σ curve.
References
[1] BI Shi-wen, YU Hai-yan. Production technology of alumina [M]. Beijing: Chemical Industry Press, 2006: 250-251. (in Chinese)
[2] ZHANG Cheng-zhong. Research on additive for inhibiting secondary reaction in clinker leaching process [D]. Shenyang: Northeastern University, 2008: 49-50. (in Chinese)
[3] HUANG Chuan-bing, WANG Yu-hua. Removal of aluminosilicates from diasporic-bauxite by selective flocculation using sodium polyacrylate [J]. Separation and Purification Technology, 2008, 59: 299-303.
[4] WANG Yu-hua, HUANG Chuan-bing, HU Yue-hua, HU Ye-min, LAN Ye. Beneficiation of diasporic-bauxite ore by selective flocculation with a polyacrylateflocculant [J]. Minerals Engineering, 2008, 21: 664-672.
[5] REN Gen-kuan. Technical research of secondary reaction inhibitor and sweetening process [J]. Light Metals, 2008(5): 16-18. (in Chinese)
[6] LI Tai-chang. Technical research of secondary reaction inhibitor and it's sweetening process [J]. Nonferrous Metals, 2002(1): 26-28. (in Chinese)
[7] SHEU E Y, STORM D A, SHIELDS M B. Adsorption kinetics of asphaltenes at toluene/acid solution interface [J]. Fuel, 1995, 74: 1475-1479.
[8] YU Hai-yan, PAN Xiao-lin, LU Zhong-ke, DING Ting-ting. Adsorption of polyethylene glycol at the interface of dicalcium silicate-sodium aluminate solution [J]. Light Metals, 2011: 251-254.
[9] OLOF S, TOBIAS H, TORGNY S, THOMAS A. Adsorption of delmopinol at the solid/liquid interface—The role of the acid–base equilibrium [J]. Journal of Colloid and Interface Science, 2010, 350: 275-281.
[10] PAUL J, JOHN R. The adsorption of a polysaccharide at the talc–aqueous solution interface [J]. Colloids and Surfaces A, 1998, 139: 27-40.
[11] WU Z S, LI C. Kinetics and thermodynamics of b-carotene and chlorophyll adsorption onto acid-activated bentonite from Xinjiang in xylene solution [J]. Journal of Hazardous Materials, 2009, 171: 582-587.
[12] SOMASUNDARAN P, KRISHNAKUMAR S. Adsorption of surfactants and polymers at the solid-liquid interface [J]. Colloids and Surfaces A, 1997, 123-124: 491-513.
[13] WANG Yan-ji, SONG Zeng-fu. Spectroscopy and chromatography [M]. Beijing: Peking University Press, 1995: 225-239. (in Chinese)
[14] HU Ying. Modern chemical and engineering thermodynamics [M]. Shanghai: Shanghai Science and Technology Press, 1994: 239-240. (in Chinese)
[15] TAYLOR J J, SIGMUND W M. Adsorption of sodium polyacrylate in high solids loading calcium carbonate slurries [J]. Journal of Colloid and Interface Science, 2010, 341: 298-302.
聚丙烯酸钠在硅酸二钙-铝酸钠溶液界面的吸附行为
于海燕, 潘晓林, 丁婷婷, 张 武, 刘 涵, 毕诗文
东北大学 材料与冶金学院,沈阳 110004
摘 要:测定了80 °C时2CaO·SiO2 (C2S)在铝酸钠溶液中吸附聚丙烯酸钠(AY)的吸附量等温线。结果表明:AY在C2S表面的吸附类型为饱和吸附,吸附行为属“L”型,符合Langmuir方程单分子层吸附模型。结合C2S的比表面积可求出AY饱和吸附时表面覆盖度为1.06 mol/μm2。同时,运用Gibbs公式处理C2S在固-液界面的吸附结果,研究吸附膜的表面压力和分子面积的关系曲线,并讨论因吸附引起的固-液界面能变化规律以及曲线与吸附类型的关系。
关键词:铝酸钠溶液;聚丙烯酸钠;硅酸二钙;界面;吸附
(Edited by YANG Hua)
Foundation item: Project (50974036) supported by the National Natural Science Foundation of China
Corresponding author: YU Hai-yan; Tel: +86-24-83686460; E-mail: yuhy@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61015-7