
Microstructure of in situ Al3Ti/6351Al composites fabricated
with electromagnetic stirring and fluxes
LI Gui-rong(李桂荣), WANG Hong-ming(王宏明), ZHAO Yu-tao(赵玉涛),
CHEN Deng-bin(陈登斌), CHEN Gang(陈 刚), CHENG Xiao-nong(程晓农)
Institute of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, China
Received 27 February 2009; accepted 25 August 2009
Abstract: The 6351 wrought aluminum alloy and K2TiF6-CaF2-LiCl components were selected as raw materials to fabricate in situ Al3Ti particulate reinforced aluminum alloy at 720 ℃ via direct melt reaction method with electromagnetic stirring (EMS). CaF2 and LiCl acted as fluxes to lower the reaction temperature of the system. It is shown that the electromagnetic stirring and fluxes accelerate the emulsion process of K2TiF6. Optical microscopy, scanning electron microscopy, transmission electron microscopy and energy dispersive spectrum were utilized to analyze the microstructure and components of composites. Compared to composites fabricated without EMS and fluxes, the sizes of endogenetic Al3Ti are refined from 10-15 μm to 2-4 μm, which are often accompanied with silicon element. The morphology of Al3Ti or Al3TiSi0.22 exhibits triangle, quadrilateral and other clumpy patterns. Because of the Ca elements from CaF2, the sizes of Mg2Si decrease from 8-10 μm to 1-2 μm due to the formation of Ca2Si.
Key words: 6351 Al alloy; microstructure; in situ particle reinforced aluminum composites; electromagnetic stirring; fluxes
1 Introduction
In recent years, the demands for new materials with excellent comprehensive properties become stronger and stronger[1-3]. The necessary characteristics of advanced functional and structural materials include high specific modulus, strength, hardness, ductility, corrosion resistance, low heat expansion coefficient, abradability and so on[4-5]. Over the past twenty years, considerable attentions have been paid to the particles reinforced metal matrix composites (abbreviated PRMMCs), especially the aluminum matrix ones which were developed rapidly due to their good performances and competitive cost[6-7]. The aerospace, automotive industries and other structural applications promote their developments, too.
Among all the reinforced particles, transition-metal trialuminide intermetallics such as Al3Zr and Al3Ti are good candidates for the in situ reinforcements of light metal matrix[8-9], which have low densities and high elastic modulus. YIN et al[10] selected Al-TiO2 to fabricate Al3Ti/Al at the reaction temperature of 1 000-1 200 ℃. Later, more attentions were paid to K2TiF6 as the source of Ti element in Al3Ti. Not only its low cost but also the lower reaction temperature than that of Al-TiO2 system attributes to the decrease of the sizes of Al3Ti particles. 6351 wrought aluminum has been utilized in the architecture, automobile, pipes and so on due to its middle strength and high elongation (T6 condition, tensile strength σb=310 MPa, yield strength σs=283 MPa, δ=14.2%)[11]. However, the strength is not strong enough to act as some important structures. It is necessary to explore new ways to fabricate new materials using the wrought alloy as matrix.
In this work, 6351 wrought aluminum alloy is selected as matrix and K2TiF6 salt is utilized to fabricate in situ Al3Ti reinforced 6351 aluminum wrought alloy composites with electromagnetic stirring. The main aim is to lower the temperature of reaction system and the particle size of Al3Ti. The thermodynamic process and microstructure of Al3Ti/6351 composites are studied in detail.
2 Experimental
2.1 Materials and sample preparation
The compositions of 6351 wrought aluminum alloy were (mass fraction): 0.7%-1.3% Si, 0.4%-0.8% Mg, 0.4%-0.8% Mn, <0.5% Fe, <0.2% Zn, <0.2% Ti, <0.1% Cu.
In order to reduce the initial melt temperature, CaF2 and LiCl were added as fluxes. The mass ratio of mixture K2TiF6, CaF2, LiCl is 80:15:5. The melting points of pure K2TiF6, CaF2 and LiCl are 682, 1418 and 605 ℃, separately. The mass ratios of mixture were optimized via testing melting points through semi sphere method. The melting point of batched mixture was 486 ℃, which was decreased by 29% compared to that of pure K2TiF6.
Firstly, the K2TiF6 salt (industry reagents, >99.5%) was pre-heated to dehydrate the bounded water in electric oven at 250 ℃ for 3 h. Then, it was cooled, ground and screened together with CaF2 and LiCl. At the same time, the Al alloy ingot was melted in an electric furnace under an argon atmosphere and held at 720 ℃(while without using fluxes the furnace temperature should be controlled at 800℃). Certain amount of dehydrated reactants powder covered by aluminum foils was added into the melt with graphite bell, so the in situ reaction between the aluminum alloy melt and added salts took place instantly. An electromagnetic stirrer (DJMR-1616W) was utilized to increase the mass transfer during the whole in situ reaction. The electric furnace was placed at the cavity center of stirrer, where the average magnetic induced intensity of electromagnetic stirring apparatus was 0.025 T. The reaction time was controlled as 3 min, then the subsidiary products of in situ reaction were removed and the composite melt was refined by CCl6 and stewed for several minutes. The composite melt was cast by semi continuous casting at 710-720 ℃ and cooled at 40-60 ℃/s. The diameter of the billet was 100 mm.
2.2 Characterization
The sample was ground, polished and tectorial membraned so as to observe the microstructure clearly. LEICA DM 2500M optical microscope (OM), JSM-7001F scanning electronic microscope (SEM), Inca Energy 350 energy dispersive spectrum (EDS), JEOL-JEM-2100-HR transmission electronic microscope (TEM) and D/max-2500PC X-ray diffractometer (XRD) were used to observe the microstructure and analyze the phases in composites. STA449C DSC (differential scanning calorimetry) was utilized to analyze the thermal effect of the chemical reaction between Al powder and K2TiF6, at a heating rate of 5 ℃/min without protective atmosphere.
The theoretical volume fraction of Al3Ti particles was set as 3% (volume fraction). The recovery rate of K2TiF4 was presumed as 90%. The mass fraction of added K2TiF6 to the matrix is 7.68%. The elementary components in composites were nominated as: 1.1%Si- 0.67%Mg-0.4%Mn-1.2%Ti-0.7%Ca-0.2%Cu-0.02%Cr- 0.01%Fe-Al (Bal.). The mass ratio of Mg to Si was 0.61. It was less than 1.73, which was the mass ratio of Mg to Si in Mg2Si particle. The mass fraction and volume fraction of Mg2Si reinforced particles in the matrix were 1.11% and 1.69%, respectively.
3 Results and discussion
3.1 Thermodynamics and kinetics of in situ reaction
The integral chemical reaction between K2TiF6 and molten Al is deduced as
13Al+3K2TiF6=3Al3Ti+K3AlF6+3KAlF4 (1)
According to the second law of thermodynamics, the Gibbs free energy ΔG of the reaction system can be expressed as:
?G=?H-T?S (2)
where H, S and T stand for the enthalpy, entropy and thermodynamic temperature respectively. At certain temperature, the ΔGT can be expressed as:
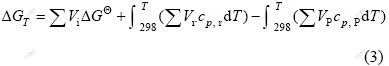
where GΘ is the standard Gibbs free energy, cp, r and cp, P are the specific heat capacity at constant pressure of reactants and products, respectively; Vr and VP are the mole volume fraction of reactants and products, respectively. Some thermodynamic parameters of involved substances in Eq.(1) are listed in Table 1.
Table 1 Some thermodynamic parameters of reactants and products in Eq.(1)
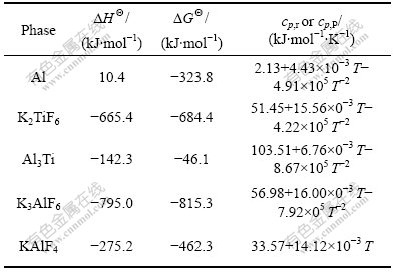
Accordingly, the ΔGT of in situ reaction of Al-K2TiF6 components can be calculated as
ΔGT =-15489.45+12.87T+0.13×10-3T2-1.94×105T-1 (4)
When the system temperature is 993 K (720 ℃), the corresponding ΔGT is -186 kJ/mol, so the in situ chemical reaction between Al and K2TiF6 salt will take place spontaneously.
Fig.1 shows the DSC curve of the thermo process of Al-10%K2TiF6 (mass fraction). At 452.4 ℃ and 606.3 ℃, there exists an apparent exothermal peak respectively. While from 796.3 ℃ the slope coefficient is larger than the heating-up velocity, which demonstrates that there is an exothermal reaction. Connecting the ΔGΘ values the three exothermal reactions are deduced as
2Al+3K2TiF6=2K3AlF6+6[F]+3[Ti] (at 452.4 ℃) (5)
2Al+K2TiF6+2[F]=2KAlF4+[Ti] (at 606.3℃) (6)
3Al+[Ti]=Al3Ti (7)
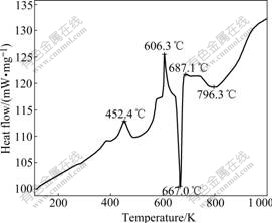
Fig.1 DSC curve of Al-10%K2TiF6
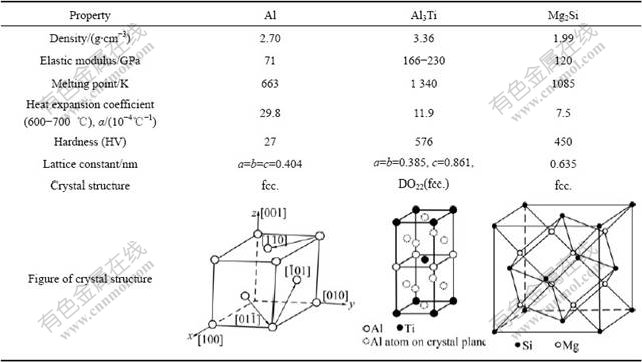
Among the products, intermetallic compound Al3Ti is the reinforced particle. Some properties of Al3Ti and other pure substances in the composites are listed in Table 2.
Table 2 Some properties of phases in composites
Al3Ti in the as-cast composites comes from two ways. One is the resultant of chemical reaction as Eq.(1), the other is the precipitate of molten Ti and Al atoms during solidification. The process can be illustrated in Fig.2.
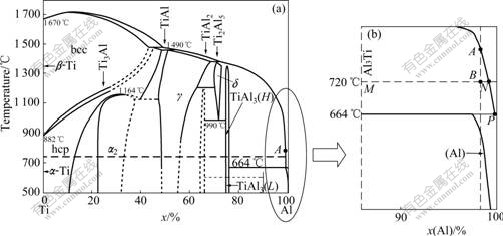
Fig.2 Phase diagram of Al-Ti binary alloy: (a) Al-Ti binary phase; (b) Partial magnification diagram of Fig.2(a)
When the volume fraction of Al3Ti in melt is 3%, the mole ratio of Ti element to Al element is about 2:98.
The component is shown as point A in Fig.2(a). The corresponding liquidus temperature is about 900 ℃. In the in situ process, the melt is stable at 720 ℃ (point B) when the solid Al3Ti precipitates. If the system is at equilibrium state, the relationship between precipitated Al3Ti and alloy melt can be expressed as

Most titanium stays in the melt, and the precipitation process can be explained as

With the decrease of melt temperature, the precipitated amount of solid Al3Ti increases, which can be calculated along the ANP curve in Fig.2(b). The process proceeds till to 664 ℃.
At 664 ℃, the peritectic reaction takes place as

The aluminum melt solidifies the surrounding solid Al3Ti. In the other words, the precipitated Al3Ti becomes the nucleus of primary aluminum. The whole process can be illustrated by Fig.3.
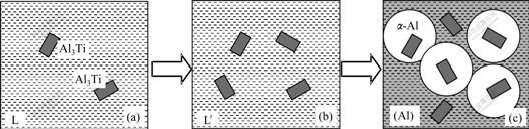
Fig.3 Evolution process of Al3Ti particles from 720 ℃ to 664 ℃: (a) Melt at 720 ℃; (b) Cooling from 720 ℃ to 664 ℃; (c) Peritectic reaction from 664 ℃
The behavior of Al3Ti formation can be disintegrated into two steps. The first is the nucleation. Electromagnetic stirring and high inner energy lead to apparent component fluctuation in melt. So in some certain position the amount of Ti is higher than the average level, which satisfies the nucleus condition of Al3Ti. The second is the growing up of Al3Ti nucleus, whether the Al3Ti phases grow up is subject to the diffusion velocity of Ti element.
3.2 Microstructure of Al3Ti reinforced phases
In Al3Ti/6351 composites, Al3Ti, β-Mg2Si, Si are all the reinforced phases, among which the volume fraction of Al3Ti is the highest. Fig.4 shows the morphology of Al3Ti particles synthesized without and with EMS and fluxes.
In Fig.4(a) the morphology of Al3Ti exhibits triangle, quadrilateral and other clumpy shapes, and the sizes of the blocks are in the range of 10-15 μm. In Fig.4(b), the morphology of Al3Ti does not change obviously, but the sizes of Al3Ti are refined from 10-15 μm to 2-4 μm. When doped K2TiF6 salt with CaF2 and LiCl, the system temperature decreases from 800 ℃ to 720 ℃. Consequently, the diffusion velocity of Ti element is restricted, which prevents the Al3Ti from growing[12]. The most important reason is that the electromagnetic stirring force and fluxes addition accelerate the emulsion process of K2TiF6. As a matter of fact, the K2TiF6 powder enters into the melt via emulsion process. The melting point (Tm) of the mixture K2TiF6-15%CaF2-5%LiCl is 486 ℃, which is much lower than that of K2TiF6 salt (the Tm of pure K2TiF6 is 682 ℃[13]). At the initial stage of the in situ reaction, the fluxes help the K2TiF6 emulsify and react with the molten Al, which enhances the recovery rate of K2TiF6 salt. The solid–liquid reaction has been partly changed into a liquid-liquid one. The electromagnetic stirring forces further improve the entering condition of emulsified salt into melt and the dynamics condition of in situ reaction between Al and K2TiF6 components. So the nucleation rate of Al3Ti particles is accelerated and the sizes of particles are refined. The rapid emulsion can be illustrated in Fig.5.
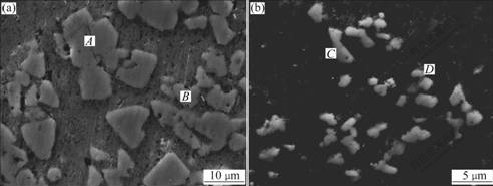
Fig.4 Morphology of Al3Ti phases in as-cast composites fabricated without (a) and with (b) EMS and fluxes
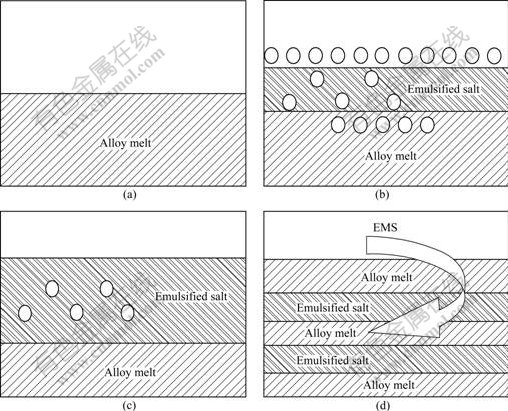
Fig.5 Schematic diagram of emulsion process of K2TiF6 salt when using EMS and fluxes (○ means K2TiF6 particle): (a) No slat; (b) Adding salt without fluxes; (c) Adding salt with fluxes; (d) With EMS and fluxes
Based on the EDS analysis, it is found that the extra silicon element gathers around the nuclei of Al3Ti. Fig.6 shows the EDS spectra of some phases in Fig.4.
It can be seen that the components of clumpy phases are different. The components of comparatively bigger ones include Al, Ti and Si elements. From the element ratio listed in Fig.6(a), it is inferred that the phases are Al3TiSix, while the components of comparatively smaller phases consist of Al and Ti elements. From the element ratio listed in Fig.6(b), it is deduced that the phases are Al3Ti. Considering the relationship between the components and the sizes of clumpy phases, it is presumed that the extra silicon elements in the melt are likely to adhere to the formed Al3Ti and promote the growth of Al3Ti.
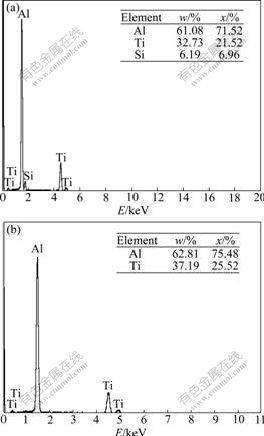
Fig.6 EDS spectra of different phases in Al3Ti/6351 composites shown in Fig.4: (a) For A, C areas: AlTiSi eutectic phases; (b) For B, D areas: AlTi phases
The reaction between Al3Ti and Si can be expressed as
Al3Ti+3xSi→TiAl3Si3x (11)
Form EDS result the value of x is deduced as 0.073. Eq.(11) can be turned into
Al3Ti+0.22Si→TiAl3Si0.22 (12)
Fig.7 shows the XRD pattern of Al3Ti/6351 composites. It demonstrates that the phases in the as-cast composites are α(Al), Al3Ti and Mg2Si. The amount of extra silicon is not large enough to be detected.
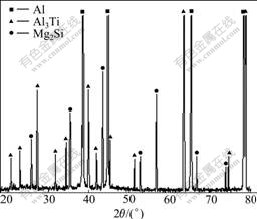
Fig.7 XRD pattern of Al3Ti/6351 composites
Fig.8 indicates the TEM morphology of Al3Ti phase in the as-cast composites. The interface between Al matrix and Al3Ti particles is clear, where is no unexpected subsidiary product. The bonding state is helpful to increase the mechanical properties of composites.
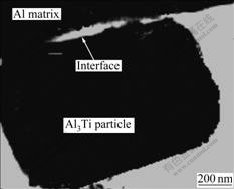
Fig.8 TEM morphology of Al3Ti phase in as-cast composites
3.3 Microstructure of Mg2Si eutectic phases
Fig.9 presents the morphologies of Mg2Si particles before and after using fluxes. It is shown that without fluxes the Mg2Si phase exhibits as Chinese character or dendrite[14]. The size of Mg2Si phase is 8-10 μm. Nevertheless, the patterns of Mg2Si particles change into polygonal shapes in composites fabricated with fluxes [15]. Meanwhile, the Mg2Si particles often combine oxygen or ferrum elements together. The average size of Mg2Si particles is 1-2 μm.
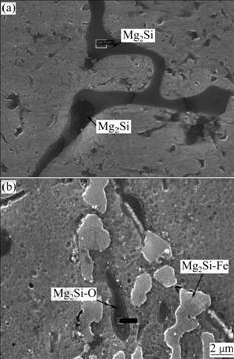
Fig.9 Morphologies of Mg2Si phases in Al3Ti/6351composites: (a) Without fluxes; (b) With fluxes
The refinement effects on Mg2Si can be explained from two views. One is due to the modification effects of. K2TiF6. The anisotropic growth of Mg2Si during solidification is suppressed by K2TiF6 as modifier[16]. The effect is caused either by poisoning the surface of Mg2Si nuclei through potassium (K) segregation at the liquid–solid interface or by changing the surface energy of Mg2Si crystals via lattice distortion due to the insertion of potassium (K) in Mg2Si lattice[17].
The other reason is due to the formation of Ca2Si. The Ca atom is released because the CaF2 reacts with molten Al,which can be illustrated as:
CaF2=Ca2++F- (13)
2F-+(2/3)Al= (2/3)AlF3+2e- (14)
Ca2++2e-=Ca (15)
The Ca2Si phases generate through the combination reaction between calcium and silicon atoms as:
2Ca+Si=Ca2Si (16)
The melting point of Ca2Si substance which becomes the nuclei of primary Mg2Si particles is above 900 ℃[18-19]. In the crystal cell of Mg2Si, the bonding force of Mg-Si is stronger than that of Si-Si, which is beneficial for Mg2Si particles to precipitate[20].
4 Conclusions
1) The Al3Ti particles reinforced 6351 aluminum matrix composites were fabricated by K2TiF6-CaF2-LiCl components at 720 ℃ via direct melt reaction method with electromagnetic stirring. The CaF2 and LiCl acted as fluxes to accelerate the emulsion process of K2TiF6 and decrease the in situ reaction temperature from 800 ℃ to 720 ℃.
2) The reinforced phases in the composites are Al3Ti, Al3TiSi0.22 and Mg2Si. Compared to composites fabricated without EMS and fluxes, the sizes of endogenetic Al3Ti or Al3TiSi0.22 particles are refined from 10-15 μm to 2-4 μm. The morphology of Al3Ti particles exhibits triangle, quadrilateral and other clumpy patterns.
3) The size of Mg2Si phases is decreased from 8-10 μm to 1-2 μm due to the modification effect of K2TiF6 salt and the formation of Ca2Si.
References
[1] LI Gui-rong, ZHAO Yu-tao, DAI Qi-xun. Fabrication and properties of particles reinforced aluminum matrix composites in-situ synthesized in Al-Zr-O-B system [J]. J Mater Sci, 2007, 42(14): 5442-5447.
[2] TJONG S C. Microstructure and mechanical characteristics of in situ metal matrix composites [J]. Mater Sci Eng A, 2000, 29: 49-113.
[3] UNLUM B S, ATIK E. Tribological properties of journal bearings manufactured from particle reinforced Al composites [J]. Mater Design, 2009, 30(4): 1381-1385.
[4] WANG Hong-ming, LI Gui-rong, ZHAO Yu-tao. Wear behavior of (Al3Zr+Al2O3)p/A359 composites by in situ electromagnetic casting [J]. Rare Metal Mat Eng, 2006, 35(4): 669-672. (in Chinese)
[5] WANG Y, WANG H Y, XIU K. Fabrication of TiB2 particulate reinforced magnesium matrix composites by two step processing method [J]. Mater Lett, 2006, 60: 1533-1537.
[6] ISIL K. Production of TiC reinforced-aluminum composites with the addition of elemental carbon [J]. Mater Lett, 2005, 59: 3795- 3800.
[7] TJONG S C, WANG G S, MAI Y W. High cycle fatigue response of in situ Al-based composites containing TiB2 and Al2O3 submicron particles [J]. Compos Sci Technol, 2005, 65: 1391-1400.
[8] VARIN R A. Intermetallic-reinforced light-metal matrix in-situ composites [J]. Metall Mater Trans A, 2002, 33(1): 193-197.
[9] LI Gui-rong, ZHAO Yu-tao, WANG Hong-ming, CHEN Gang, DAI Qi-xun, CHENG Xiao-nong. Fabrication and properties of in situ (Al3Zr+Al2O3)p/A356 composites cast by permanent mould and squeeze casting [J]. J Alloys Compd, 2009, 471(1/2): 530-535.
[10] YIN Yu-juan, ZHAO Yu-hou, XIA Yong-xi. Study status of strengthening phase of in-situ aluminum matrix composites [J]. Heat Process Technol, 2006, 35(7): 70-73. (in Chinese)
[11] DURMU? H K, OZKAYA E, MERI? C. The use of neural networks for the prediction of wear loss and surface roughness of AA 6351 aluminium alloy [J]. Mater Design, 2006, 27(2): 156- 159.
[12] QIN Q D, ZHAO Y G. Nonfaceted growth of intermetallic Mg2Si in Al melt during rapid solidification [J]. J Alloy Compd, 2008, 462(1/2): L28-L31.
[13] WANG Hong-ming, LI Gui-rong, DAI Qi-xun, LEI Yu-cheng, ZHAO Yu-tao, LI Bo, SHI Guo-min, REN Zhong-ming. Effect of additives on viscosity of LATS refining ladle slag [J]. ISIJ Int, 2006, 46(5): 637-640.
[14] PANIGRAHI S K, JAYAGANTHAN R. Effect of cryorolling on microstructure of Al-Mg-Si alloy [J]. Mater Lett, 2008, 62: 2626- 2629.
[15] VARIN R A. Intermetallic-reinforced light-metal matrix in-situ composites [J]. Metall Master Trans A, 2002, 33(1): 193-197.
[16] ZHAO Y G, QIN Q D, ZHAO Y Q. In situ Mg2Si/Al-Si composites modified by K2TiF6 [J]. Mater Lett, 2004, 58: 2191-2194.
[17] HADIAN R, EMAMY M, VARAHRAM N, NEMATI N. The effect of Li on the tensile properties of cast Al-Mg2Si metal matrix composite [J]. Mater Sci Eng A, 2008, 490(1/2): 250-257.
[18] TANI J, KIDO H. Lattice dynamics of Mg2Si and Mg2Ge compounds from first-principles calculations [J]. Comp Mater Sci, 2008, 42: 531-536.
[19] TAKAGI N, SATO Y, MATSUYAMA T, TATSUOKA H, TANAKA M, FENGMIN C, KUWABARA H. Growth and structural properties of Mg2Si and Ca2Si bulk crystals [J]. Applied Surface Sci, 2005, 244(1/4): 330-333.
[20] GAO Ying-jun, LI Yun-wen, WANG Tai-cheng, HUANG Chuan-gao, GOU Xian-hua. Keymat analysis of reinforcement effect of Al-Mg-Si alloy [J]. Light Metal, 2005, (2): 55-57. (in Chinese)
Foundation item: Project(2007AA03Z548) supported by the National High-Tech Research and Development Program of China; Project(207038) supported by the Key Program of Ministry of Education of China; Project(06-D-021) supported by the Talents Peak in Six Key Fields of Jiangsu Province in China; Project(07JDG084) supported by the Technical Enablement Foundation for the Super Special Talents of Jiangsu University; Project(20071108) supported by the Technical Enablement Foundation of Ministry of Education for the Returned Scholars; Project(20060299006) supported by the PhD Programs Foundation of Ministry of Education of China
Corresponding authors: ZHAO Yu-tao; Tel: +86-511-88797658; E-mail: zhaoyt@ujs.edu.cn; LI Gui-rong; Tel: +86-13951408072; E-mail: whmlgr@ujs. edu.cn
DOI: 10.1016/S1003-6326(09)60181-3
(Edited by FANG Jing-hua)