J. Cent. South Univ. Technol. (2010) 17: 522-528
DOI: 10.1007/s11771-010-0517-1
Characteristics of extracellular fluorescent substances of
aerobic granular sludge in pilot-scale sequencing batch reactor
TU Xiang(涂响), SU Ben-sheng(苏本生), LI Xiao-ning(李小宁), ZHU Jian-rong(竺建荣)
School of Environment, Beijing Normal University, Beijing 100875, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: The aerobic granular sludge was cultivated in a pilot-scale sequencing batch reactor (SBR), and some of the granules were stored at 8 ℃ for 150 d. Extracellular polymeric substances (EPS) of sludge samples were extracted and analyzed during the granulation and storage process. The results show that the contents of protein and EPS increase along with the granulation process, while polysaccharides remain almost unchanged. The content of protein in EPS is almost two-fold larger than that of polysaccharides in granular sludge cultivated with municipal wastewater. Moreover, some of the granules disintegrate during storage, corresponding to the decrease of protein contents in EPS. Three peaks are identified in three-dimensional excitation emission matrix (EEM) fluorescence spectra of the EPS in the aerobic granules. Two peaks (A and B) are attributed to the protein-like fluorophores, and the third (peak C) is related to visible fulvic-like substances. Peak A gradually disappears during storage, while a new peak related to ultraviolet fulvic acid (peak D) is formed. The formation and the stability of aerobic granules are closely dependent on the quantity and composition of EPS proteins. Peak C has no obvious changes during granulation, while the fulvic-like substances present an increase in fluorescence intensities during storage, accompanied with an increase in structural complexity. The fulvic-like substances are also associated with the disintegration of the aerobic granules.
Key words: aerobic granules; extracellular polymeric substances (EPS); protein; fulvic acid; sequencing batch reactor (SBR); excitation emission matrix fluorescence spectroscopy
1 Introduction
Granulation of aerobic activated sludge, a novel environmental biotechnology, is increasingly drawing interest in the area of biological wastewater treatment. Aerobic granules have many advantages over conventional bioflocs, such as excellent settling ability, high biomass retention, and good resistance to inhibitory and toxic compounds [1]. Extracellular polymeric substances (EPS), which are released from microorganism growth and cell lysis [2], have played a crucial role in promoting aerobic granulation and maintaining structural integrity of granular sludge [3]. The major components of EPS include polysaccharides, proteins, humic acids, phospholipids and nucleic acids [4], which help to initiate the formation of microbial aggregates, adhesion to surfaces and flocculation [5].
Many attempts were made to explore the biochemical composition of EPS with the application of numerous innovative analytical instruments. Fluorescence spectrometry is one of the effective options [4, 6-7]. EPS contain large quantities of aromatic structures and unsaturated fatty chains with various types of functional groups [5]. Their intrinsic fluorescence characteristics can provide information concerning the structure, configuration and heterogeneity of the components in sludge EPS [8]. However, since the biological wastewater treatment reactor is a complex system, it is difficult to unveil the entire fluorescence property of the EPS.
Three-dimensional excitation emission matrix (EEM) fluorescence spectroscopy is a rapid, selective and sensitive technique. Its advantage is that information regarding the fluorescence characteristics can be completely acquired by changing the excitation wavelength and emission wavelength simultaneously [9]. It has been proven to be useful to differentiate the transformations of organic matters in natural environments and bioreactors according to the fluorescence of different spectral regions [9-12]. Therefore, EEM fluorescence spectroscopy can be employed to obtain a large amount of information about changes in structural and functional properties of EPS during the biogranulation process.
Recently, ADAV et al [13] and NI et al [14] have cultivated aerobic granular sludge in pilot-scale sequencing batch reactor (SBR) with practical wastewater. However, the research on properties of sludge EPS during granulation process with municipal wastewater has been hardly carried out. The purposes of this work were, therefore, to obtain the EPS characteristics at different operation time in a pilot SBR and contribute to a better understanding of the mechanism in biogranulation process with the assistant of EEM fluorescence technology. The property changes of sludge EPS extracted from granules at different storage time were also measured to investigate the relationship between EPS and structure stability of aerobic granules.
2 Experimental
2.1 Pilot SBR operation
A 6 m3 SBR plant was fed with municipal wastewater and operated continuously for 150 d. Flocculent sludge was seeded to the SBR for cultivation of aerobic granules. The reactor was set in a 6 h cycle, with 30 min feeding, 50 min anoxic, 230 min aeration, 20 min sedimentation and 30 min effluent withdrawal. The exchange ratio was maintained at 65% during the entire operating period. After 150 d operation, 20 L mixed liquor was taken out from the reactor and stored in a refrigerator at 8 ℃ for 150 d.
2.2 Extraction of EPS and chemical analysis
During the SBR operation, sludge samples were obtained at the end of each SBR cycle for analysis. EPS were extracted from the sludge samples every 30 d during SBR operation and storage, according to the thermal treatment method described by CHANG and LEE [15]. The samples were centrifuged at 3 200 r/min for 30 min. After discarding the supernatant, the remaining sludge was washed and re-suspended with saline water (0.9% NaCl solution). The mixed liquor was then subjected to heat treatment (100 ℃, 1 h) and centrifuged again under the same operating conditions. The supernatant was filtrated through a 0.45 mm membrane and the filtrate was collected as EPS solution.
The total organic carbon (TOC) concentration of the EPS solution was measured as the EPS concentration (TOC analyzer, Shimadzu, Japan). Proteins (PN) and polysaccharides (PS) contents were quantified following the method described by LOWRY et al [16] and the phenol-sulfuric acid method described by DUBOIS et al [17], respectively. Particle size analysis of the sludge was carried out by using laser diffraction spectroscope (Mastersizer, Malvern 2000, UK). Chemical oxygen demand (COD), sludge volume index (SVI) and mixed liquor suspended solid (MLSS) were determined according to the standard method in Ref.[18].
2.3 EEM fluorescence measurements
EPS solutions were extracted from 2.0 g dry sludge samples for fluorescence analysis. The EEM fluorescence determinations were made using a Perkin- Elmer LS50B luminescence spectrometer equipped with the FL WINLAB 5.00.02 for data processing. Excitation- emission fluorescence matrix (EEFM) was created by measuring fluorescence intensity across excitation wavelengths ranging from 200 to 450 nm and emission wavelengths ranging from 280 to 550 nm. The scan speed of 1 200 nm/min was selected with a slit-width of 10 nm, a PMT voltage of 700 V and a response of 0.5 s.
3 Results and discussion
3.1 Evolution of sludge morphology
The sludge properties, including MLSS content and mean particle size, are shown in Fig.1. The seeding sludge had a mean floc size of 80 ?m with a fluffy and irregular structure. During the first 80 d of operation, there was a slow increase in MLSS content from 3.5 to 4.4 g/L and the sludge morphology was not significantly improved as well. On day 90, tiny granular sludge was observed at the bottom of the reactor, since then the number and average diameter of the granules kept increasing. Meanwhile, the MLSS content began to increase remarkably and became stable at 7.8 g/L. Mature granules were obtained on day 150, with a compact structure and a mean size of 275 ?m. In addition, the formation of aerobic granules led to great settling ability and high COD removal efficiencies of 90%-95%.

Fig.1 Variations of particle size and content of MLSS during granulation and storage
Aerobic granules remained a spherical shape after 150 d storage, whereas the apparent color of granules turned from brow-yellow to dark-black, as a result of sulfide generation by sulphate-reducing bacteria. As shown in Fig.1, during the first 90 d of storage, the average particle size of the granular sludge fluctuated in a range of 260-268 ?m. Thereafter, it decreased evidently from 251 to 190 ?m in the following 60 d. Moreover, the SVI value increased from 30 to 75 mL/g during 150 d, indicating that some of the granules disintegrated and the sludge settling ability gradually decreased after an extended storage time. On the other hand, the MLSS concentration was reduced to 6.4 g/L after 150 d storage. This suggests that without substrate addition, the mass of active bacteria decreases due to processes such as decay, maintenance, endogenous respiration and lysis.
3.2 EPS characterization during granulation
To assess the role of EPS secreted by bacteria during aerobic granulation, the content of EPS, extracellular PN and PS contents were measured at different operation time. It is presented in Fig.2 that, the EPS concentration increased from 76.6 mg/L in seed sludge to 131.5 mg/L in the mature granules. Similar to the trend of EPS content, the PN content increased significantly within the first 90 d, then it maintained at 86-88 mg/g when the aerobic granules formed. The amount of PS extracted from seed sludge was initially greater than that of PN, while it had no clear increment throughout the granulation process in the SBR. Finally, the PN content in EPS of the granules was 1.7-1.8 times higher than that of PS, indicating that the aerobic granules cultivated in municipal wastewater consisted of higher protein levels in EPS than those in the floc sludge.
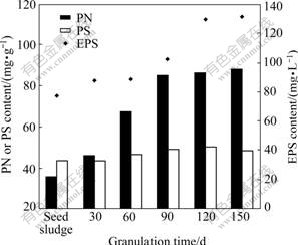
Fig.2 Changes in PN, PS and EPS contents during granulation process
The three-dimensional EEM spectra of sludge EPS along with the granulation process are shown in Fig.3. Three peaks are identified from the EEM fluorescence spectra. The first peak is located at the excitation/ emission wavelengths (λEx/λEm) of 220 nm/345 nm (peak A), while the second main peak is observed at the λEx/λEm of 280 nm/345 nm (peak B). The two peaks are attributed
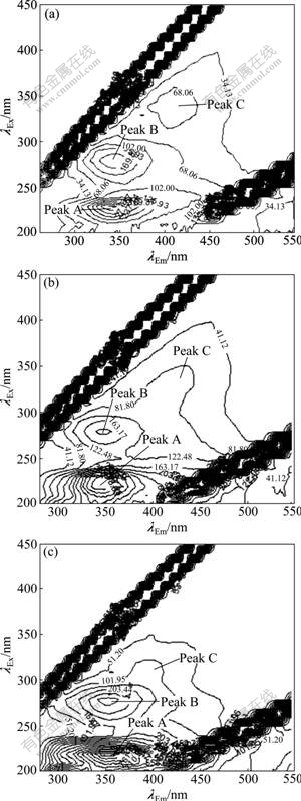
Fig.3 Three-dimensional EEM spectra of sludge of EPS during granulation process after different operation time: (a) 30 d; (b) 90 d; (c) 150 d
to the protein fluorescence in which peak A is reported as simple aromatic protein-like substances and peak B is associated with the tryptophan protein-like substances [19]. The third peak at around λEx/λEm of 330 nm/420 nm (peak C) is described as the fluorescence of visible fulvic-like substances [20]. Since the PS has no fluorophores, there is no fluorescence of PS in the EEM spectra.
Fluorescence parameters of the spectra at different operation time during granulation are summarized in Table 1 for quantitative analysis. It is observed that locations of the three peaks are not clearly changed from day 30 to day 90, whereas fluorescence intensities of both peaks A and B increase significantly during the first 90 d, which is consistent with the increase of protein contents in sludge EPS. The intensities of peaks A and B on day 150 are 1.5 and 1.7 times higher than those of day 30, respectively. This further confirms that protein is enriched in the EPS of aerobic granules. The peak intensity ratio of peak A to peak B increases along with granulation, suggesting that types and components of the EPS proteins in aerobic granules are chemically different from those of the flocculent sludge. In addition, compared with the fluorescence signals on day 30, the locations of peaks A and B have a red shift of 10 nm in terms of emission wavelengths when the aerobic granules are formed (day 150). The red shift is related to the presence of carbonyl-containing substituents, such as alkoxyl, hydroxyl and carboxyl constituents, which indicates the increase of polymerization and structural complexity of proteins in the EPS [21]. The intensity of peak C is much weaker than that of peaks A and B. Moreover, it does not vary evidently during 150 d, suggesting that humic substances may be unrelated to the process of aerobic granulation.
It is widely accepted that the PN and amino acids are the hydrophobic components of the EPS, while the PS is hydrophilic [22]. Thus, the increase of mass ratio of PN to PS is closely correlated with an increase in the sludge hydrophobicity and a decrease in surface negative charges of bacterial cells, which may further trigger the aerobic granulation [23]. MCSWAIN et al [24] confirmed that the protein content was 50% more in the EPS of aerobic granules than that in the flocculent sludge, and their observations revealed that granule formation and stability were dependent on a noncellular protein core. ZHANG et al [22] noted that sludge surfaces were more hydrophobic and less negatively charged after aerobic granulation than the flocculent seed sludge, and the ratio of protein to polysaccharide within the sludge EPS increased with the granulation from 2.3 to 4.9, corresponding to the changes in the surface properties of the sludge. ADAV and LEE [4] also found that the mass ratio of PN to PS for sludge flocs was approximately 0.9, while the PN content was significantly enriched in the EPS of aerobic granules, producing a mass ratio of PN to PS being 3.4-6.2. Moreover, it was disproved that the EEM technique could be used as a quantitative index of extracted EPS from flocculent sludge or granules.
However, the previous literatures contained contradictory reports on the composition of EPS in biogranules, especially the mass ratio of PN to PS. LIU et al [23] also reported that the EPS are composed mainly of carbohydrates. Current evidences show that the quantity and the composition of EPS produced by bacteria depend on a number of factors, such as microbial species, growth phase, type of substrate, operating conditions of the SBR, including oxygen limitation, reactor type, settling time, culture temperature, and shear force. In fact, the component of sludge EPS can change correspondently under different cultivation conditions, while it is shown by the results of this pilot SBR operation treated with municipal wastewater that, the PN is highly associated with the formation of aerobic granules.
3.3 Characteristics of EPS during storage
WANG et al [25] and ZENG et al [26] found that aerobic granules had good structural stability and bioactivity reactivation ability after less than 120 d storage, which was consistent with the findings of this study. Meanwhile, the evolution of EPS was also investigated during the whole 150 d storage. It can be observed from Fig.4 that the EPS content fluctuated within 110-130 mg/L throughout the storage. The PS content decreased sharply from 47.5 to 21.4 mg/g in the first 30 d, and remained almost unchanged in the following days. However, a slight decrease of the PN content occurred during the first 60 d, and then the PN content was greatly reduced to 54 mg/g on day 90 and stabilized at this level until the end of storage. During the storage time, microorganisms used EPS as substrate and energy source. Meanwhile, EPS was produced by microorganisms under the stimulation of starvation and
Table 1 Fluorescence spectral parameters of EPS during granulation process

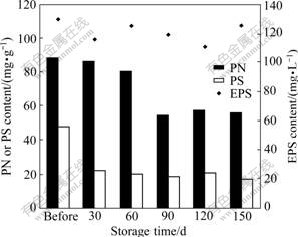
Fig.4 Changes in PN, PS and EPS contents during storage
cell lysis. This could explain the fluctuation of EPS content during storage. It is also noted that the aerobic granules began to disintegrate since the PN content in EPS decreased largely, which further implied that the protein contributes more to the bacterial aggregate structure and stability than the PS.
Fig.5 shows the EEM fluorescence spectra of EPS samples at different storage time. It is worth pointing out that a new peak (peak D) could be observed on day 30, which was located at λEx/λEm of 240 nm/420 nm and reported as ultraviolet fluorescence of fulvic-like substances. On the other hand, peak A suddenly disappeared since day 90, probably due to the break-up of protein molecules into small fragments, including the decomposition of condensed aromatic groups, the reduction of conjugated bonds in a chain structure, and elimination of particular functional groups such as carbonyl, hydroxyl, and amine [27]. The intensity of peak B also decreased continuously, corresponding to the decrease of EPS proteins throughout the 150 d storage (Table 2), which is strongly involved in the disintegration of aerobic granules. Meanwhile, the location of peak B demonstrated a red shift of 25 nm along the emission wavelengths on day 120, and it was then shifted back to 350 nm. Moreover, peaks C and D on day 150 were blue-shifted by 10-15 nm along the emission axis compared with that on day 120. These variations in peak location were probably associated with the decrease of pH value from 7.5 to 6.2 during storage [28]. The intensity of peak C on day 30 tripled compared with that of mature granules (Table 1), and the peak intensity ratio of peak C to peak D also kept increasing
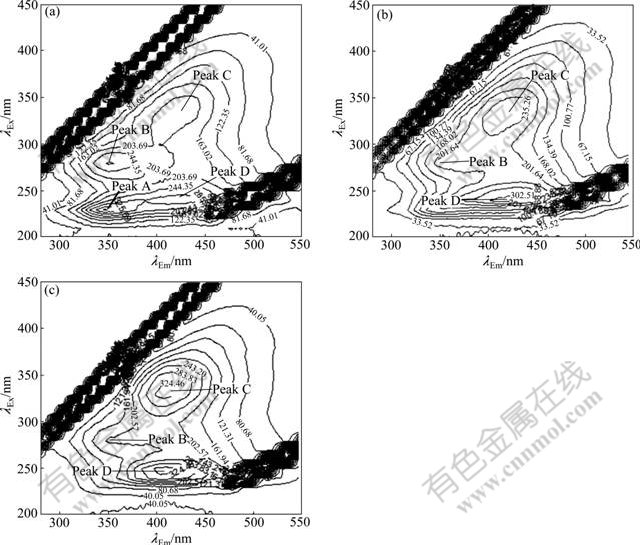
Fig.5 Three-dimensional EEM spectra of EPS during different storage time: (a) 30 d; (b) 90 d; (c) 150 d
Table 2 Fluorescence spectral parameters of EPS during storage
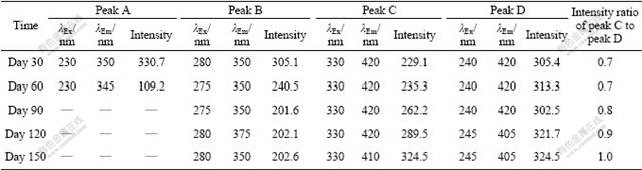
during storage. These indicate that the soluble fulvic substances with larger relative molecular mass and higher degree of aromatization become dominant in the EPS, which is unfavorable for the biomass activity and stability of the aerobic granules.
4 Conclusions
(1) Aerobic granular sludge with an average particle size of 275 ?m is cultivated in pilot SBR after 150 d operation. Formation of aerobic granules is coupled to an increase of both EPS and extracellular protein contents. The protein content decreases during 150 d storage, corresponding to the decrease of particle size. Proteins are more dominant than polysaccharides in the EPS of mature granules, and play a crucial role in formation and maintaining structural integrity of aerobic granules.
(2) Three peaks are identified in EEM fluorescence spectra for the EPS of the aerobic granules. Two peaks (A and B) are related to the protein-like substances, and the third (peak C) to visible fulvic-like substances. Peak intensities of peaks A and B have a similar changing pattern with the increase of contents of EPS proteins. Meanwhile, the red shifts of both peaks A and B indicate that the proteins have an increase in degree of structural complexity during granulation. Peak C does not change evidently during 150 d operation, suggesting that fulvic substances contribute less to the formation of aerobic granules.
(3) Peak A disappears during the storage, while peak D described as ultraviolet fluorescence of fulvic-like substances is formed. The fulvic substances keep increasing in relative molecular mass and degree of aromatization during the storage, which have negative effects on the stability of aerobic granules.
References
[1] WANG X H, ZHANG H M, YANG F L, WANG Y F, GAO M M. Long-term storage and subsequent reactivation of aerobic granules [J]. Bioresource Technology, 2008, 99(17): 8304-8309.
[2] ZHOU Jian, WU Zhi-gao, JIANG Wen-chao. Effects of extra-cellular polymeric substances on organic pollutants biodegradation kinetics for A-step of adsorption-biodegradation process [J]. Journal of Central South University of Technology, 2006, 13(3): 229-233.
[3] LI X F, LI Y J, LIU H, HUA Z Z, DU G C, CHEN J. Correlation between extracellular polymeric substances and aerobic biogranulation in membrane bioreactor [J]. Separation Purification Technology, 2008, 59(1): 26-33.
[4] ADAV S S, LEE D J. Extraction of extracellular polymeric substances from aerobic granules with compact interior structure [J]. Journal of Hazardous Materials, 2008, 154(1/3): 1120-1126.
[5] WINGENDER J, NEU T R, FLEMMING H C. Microbial extracellular polymeric substances: Characterization, structures and function [M]. Berlin: Springer, 1999.
[6] GAO Jing-feng, GUO Jian-qiu, CHEN Ran-ni, SU Kai, PENG Yong-zhen. Comparison of the efficiency of five extracellular polymeric substances (EPS) extraction methods using three- dimensional excitation and emission matrix (EEM) fluorescence spectroscopy together with chemical analysis [J]. Environmental Chemistry, 2008, 27(5): 662-668. (in Chinese)
[7] TAN Shu-zhen, LONG Shu, XIA Jiao-yun, CAO Zhong, ZHANG Ling, GONG Fu-chun. Novel fluorescence sensor based on covalent immobilization of 3-amino-9-ethylcarbazole via electrostatically assembled gold nanoparticle layer [J]. Journal of Central South University of Technology, 2009, 16(2): 212-217.
[8] SHENG G P, YU H Q. Characterization of extracellular polymeric substances of aerobic and anaerobic sludge using three-dimensional excitation and emission matrix fluorescence spectroscopy [J]. Water Research, 2006, 40(6): 1233-1239.
[9] BAKER A. Fluorescence excitation-emission matrix characterization of some sewage-impacted rivers [J]. Environment Science Technology, 2001, 35(5): 948-953.
[10] PEURAVUORI J, KOIVIKKO R, PIHLAJA K. Characterization, differentiation and classification of aquatic humic matter separated with different sorbents: Synchronous scanning fluorescence spectroscopy [J]. Water Research, 2002, 36(18): 4552-4562.
[11] WANG Z W, WU Z C, TANG S J. Characterization of dissolved organic matter in a submerged membrane bioreactor by using three-dimensional excitation and emission matrix fluorescence spectroscopy [J]. Water Research, 2009, 43(6): 1533-1540.
[12] LI W H, SHENG G H, LIU X W, YU H Q. Characterizing the extracellular and intracellular fluorescent products of activated sludge in a sequencing batch reactor [J]. Water Research, 2008, 42(12): 3173-3181.
[13] ADAV S S, LEE D J, SHOW K Y, TAY J H. Aerobic granular sludge: Recent advances [J]. Biotechnology Advances, 2008, 26(5): 411-423.
[14] NI B J, XIE W M, LIU S G, YU H Q, WANG Y Z, WANG G, DAI X L. Granulation of activated sludge in a pilot-scale sequencing batch reactor for the treatment of low-strength municipal wastewater [J]. Water Research, 2009, 43(3): 751-761.
[15] CHANG I S, LEE C H. Membrane filtration characteristics in membrane-coupled activated sludge system: The effect of physiological states of activated sludge on membrane fouling [J]. Desalination, 1998, 120(3): 221-233.
[16] LOWRY O H, ROSEBOURGH N J, FARR A R, RANDALL R J. Protein measurement with the folin phenol reagent [J]. Journal of Biological Chemistry, 1951, 193(1): 265-275.
[17] DUBOIS M, GILLES K A, HAMILTON P A. Colorimetric method for determination of sugars and related substances [J]. Analytical Chemistry, 1956, 28(3): 350-356.
[18] APHA. Standard methods for the examination of water and wastewater [M]. Washington D C: American Public Health Association, 1998.
[19] BAKER A, INVERARITY R. Protein-like fluorescence intensity as a possible tool for determining river water quality [J]. Hydrological Processes, 2004, 18(15): 2927-2945.
[20] COBLE P G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy [J]. Marine Chemistry, 1996, 51(4): 325-346.
[21] CHEN J, GU B, LE-BOEUF E J, PAN H, DAI S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions [J]. Chemosphere, 2002, 48(1): 59-68.
[22] ZHANG L L, FENG X X, ZHU N W, CHEN J M. Role of extracellular protein in the formation and stability of aerobic granules [J]. Enzyme and Microbial Technology, 2007, 41(5): 551-557.
[23] LIU Y Q, LIU Y, TAY J H. The effects of extracellular polymeric substances on the formation and stability of biogranules [J]. Applied Microbiology and Biotechnology, 2004, 65(2): 143-148.
[24] MCSWAIN B S, IRVINE R L, HAUSNER M, WILDERER P A. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge [J]. Applied and Environmental Microbiology, 2005, 71(2): 1051-1057.
[25] WANG X H, ZHANG H M, YANG F L, WANG Y F, GAO M M. Long-term storage and subsequent reactivation of aerobic granules [J]. Bioresource Technology, 2008, 99(17): 8304-8309.
[26] ZENG P, ZHUANG W Q, TAY S T L, TAY J H. The influence of storage on the morphology and physiology of phthalic acid-degrading aerobic granules [J]. Chemosphere, 2007, 69(11): 1751-1757.
[27] SWIETLIK J, DABROWSKA A, RACZYK-TANISLAWIAK U, NAWROCKI J. Reactivity of natural organic matter fractions with chlorine dioxide and ozone [J]. Water Research, 2004, 38(3): 547-558.
[28] PATEL-SORRENTINO N, MOUNIER S, BENAIM J Y. Excitation-emission fluorescence matrix to study pH influence on organic matter fluorescence in the Amazon basin rivers [J]. Water Research, 2002, 36(10): 2571-2581.
Foundation item: Project(2006AA06Z318) supported by the National High-Tech Research and Development Program of China
Received date: 2009-11-20; Accepted date: 2010-01-09
Corresponding author: ZHU Jian-rong, PhD, Professor; Tel: +86-10-58802862; E-mail: zjrthua@sohu.com
(Edited by CHEN Wei-ping)