J. Cent. South Univ. (2012) 19: 1718-1723
DOI: 10.1007/s11771-012-1198-8
Bioleaching of chalcopyrite by Leptospirillum ferriphilum
HU Ke-ting(胡可婷), GU Guo-hua(顾帼华), LI Shuang-ke(李双棵), QIU Guan-zhou(邱冠周)
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: Chalcopyrite oxidation rates were examined under various conditions in the presence of Leptospirillum ferriphilum, in which the effects of different pulp content, inoculation amount, external addition of Fe3+ and initial pH value were studied. The bioleaching residues were investigated by X-ray diffractograms (XRD), scanning electron microscopy (SEM) and energy dispersion spectrum (EDS) analysis. The results show that low pulp concentration increases the leaching rate of copper, and external addition of Fe3+ is also beneficial to leaching chalcopyrite. The changes of inoculation amount and initial pH from 1.6 to 2.5 have a little effect on the final leaching rate. The results also imply that Fe3+ ions are important for bioleaching of chalcopyrite. At the end of bioleaching, jarosite and sulfur are observed on the surface of chalcopyrite residues by using XRD, SEM and EDS. With the passivation layer formed by jarosite and sulfur, the continuous copper extraction is effectively blocked.
Key words: chalcopyrite; Leptospirillum ferriphilum; bioleaching; redox potential; jarosite
1 Introduction
Chalcopyrite, CuFeS2, is the most important copper- bearing mineral in the world. Unlike many other ores, it is known to be recalcitrant to hydrometallurgical processing [1]. Researchers have strived for decades to accelerate the speed of chalcopyrite in the biological leaching. One of the important factors for leaching tests is the selection of suitable microorganisms [2]. At present, at least eleven putative prokaryotic divisions can show this phenomenon [3-4]. Leptospirillum ferriphilum (L. ferriphilum) has recently been found to be the most representative of the prokaryotes in bio-hydrometal lurgical operations [5]. It is an aerobic Gram negative bacterium. L. ferriphilum inhabits acidic environments and derives energy from the oxidation of inorganic substances that occur naturally in ore deposits and in acid mine drainage (AMD). It shows a high affinity towards sulfide minerals [6-7]. Therefore, understanding the bioleaching rule of L. ferriphilum becomes very important and can also help us to make a further investigation of bioleaching related with other bacteria.
Bioleaching of chalcopyrite is an important industry target. The main problem hindering commercial application of chalcopyrite bioleaching is the slow dissolution rate. The polysulphides elemental sulfur layer and the iron-hydroxy precipitate layer, such as jarosite on the mineral surface, are thought to be the cause of slow dissolution rate [8-9]. The insoluble reaction products hinder greater copper extraction by restricting the flow of bacteria, nutrients, oxidants and reaction products to and from the mineral surface [10-11]. Even now, the problem of chalcopyrite passivation has not been completely solved and needs further studies.
As is known, the process of hydrometallurgical is rather complex. The bioleaching rate is determined not only by mineral’s own quality or different types of bacteria, but also by environmental conditions, such as pH, redox potential, pulp concentration, bacteria amount, other ion concentration, and temperature. Herein, the relationship between the redox potential and extraction of copper was evaluated. The effects of pulp concentration, inoculation amount, external addition of ferric ion and the initial pH value on the bioleaching of chalcopyrite were examined using the selected strains of bacteria. The surfaces of the chalcopyrite residues were analyzed by XRD, SEM and EDX. In addition, the mechanism of chalcopyrite bioleaching was studied.
2 Materials and methods
2.1 Chalcopyrite preparation
All bioleaching tests were performed with chalcopyrite mineral (approximately 90% CuFeS2, mass fraction) from Daye, Hubei Province, China. The samples were splintered into small fragments with a hammer and dry ground in a porcelain ball milling. The product was sieved to be less than 0.074 mm for bioleaching experiments. The chemical analysis showed that the chalcopyrite mainly consisted of 30.74%Cu, 30.64% Fe and 33.24%S (mass fraction).
2.2 Microorganisms and culture media
Leptospirillum ferriphilum type strain (DQ343299), provided by the Key Lab of Biometallurgy in Central South University, China, was used. It was grown in medium 9K, consisting of 3.0 g/L (NH4)2SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O and 0.01 g/L Ca(NO3)2. The initial pH value of solution with L. ferriphilum was 1.6. The temperature in the rotary shaker was 40 °C, and its rotating speed was 170 r/min. Prior to bioleaching experiments, the domesticated bacteria were transferred at least five times with the increasing concentration of chalcopyrite medium, so as to adapt the environment. Cells were harvested by centrifugation and washed twice in distilled water with pH 1.6. Cells were then suspended in basal salts medium without energy sources. The concentration of the soliquid was 1×108 cell/mL.
2.3 Bioleaching tests
All bioleaching tests were performed in 250 mL flasks containing 100 mL medium. The 9K basal salt medium without iron was used in the sulfide mineral bioleaching experiments. The inoculation amount was 5-15 mL and mineral content was 1%-5% (w/V). Fe3+ used in this test came from Fe2(SO4)3, with relative molecular mass of 399.88. The flasks were shaken in rotary shakers for 21 d. The rotating speed and temperature were 170 r/min and 40 °C, respectively.
In all bioleaching tests, every flask was sampled every 3 d by removing a 2 mL aliquot of the leaching solution, which was used for the analysis of copper ion. The redox potentials were measured using a platinum electrode combined with a Hg/HgCl2 reference electrode. The bioleaching residues were examined using X-ray diffractograms (XRD), scanning electron microscopy (SEM) and energy dispersion spectrum (EDS) analysis.
3 Results and discussion
3.1 Effect of pulp concentration
The leaching rate of copper, the redox potential and pH value with 10% inoculation of L. ferriphilum in different pulp contents are shown in Fig. 1 and Fig. 2, respectively.
Figure 1 shows increasing the pulp content results in reducing the leaching rate of chalcopyrite. The reasons for this are the high solid content of pulp hinders transferring oxygen and carbon dioxide to strains and the shear stress of mineral is harmful to bacteria, which leads to longer lag phase of strains. In addition, it can be noticed that copper extractions in different conditions are low, and the highest leaching rate is no more than 30%.
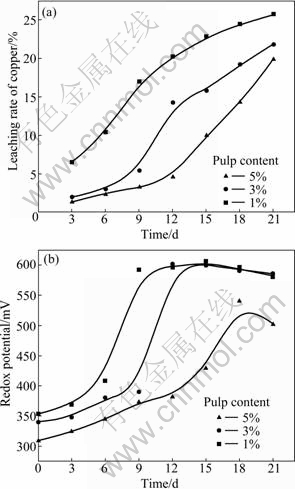
Fig. 1 Leaching rate of copper and redox potential as function of time under various pulp contents: (a) Leaching rate of copper; (b) Redox potential
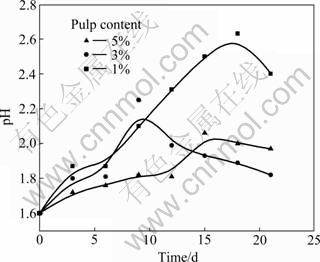
Fig. 2 pH values as function of time under different pulp contents
The pulp redox potential increases rapidly at the initial stage, mainly due to L. ferriphilum possessing the ability of oxidizing Fe2+ to Fe3+ (reaction (1)). The lower the pulp content is, the faster and the higher the potential rises at the initial phase. It is consistent with the leaching efficiency, because lower pulp density is beneficial to the growth of bacteria. The potential declines in later stage of bioleaching. This suggests that ferric ion has been consumed (reactions (2) and (3)). It can be found that there is a definite link between leaching rate and redox potential. Certain potential is beneficial to bioleaching of chalcopyrite.
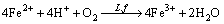 (1)
(1)
 →
→
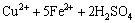 (2)
(2)
 →
→
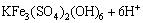 (3)
(3)
From the change of pH during bioleaching process in Fig. 2, the leaching of chalcopyrite is an acid consumption process at the early stage of bioleaching and an acid production process during the later stage. The change of pulp contents has an important influence on pH value. The higher the pulp content is, the faster the pH value rises, because dissolution of chalcopyrite is an acid consumption process (reaction (4)).
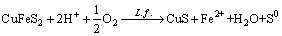 (4)
(4)
3.2 Effect of inoculation amount
Bacteria growth curves are presented in Fig. 3. The pulp content is 1%. At the initial bioleaching stage, the increasing concentration of bacteria is not significant, especially for the 5% inoculation amount with about 4 d lag phase. The logarithmic growth phase of bacteria occurs after a lag phase, and bacteria in high inoculation amount grow quickly and enter a stationary phase prior to bacteria in low inoculation amount. But the amount of bacteria in different inoculation amounts is almost the same in the end, because the nutrient substance or oxygen which can be provided to bacteria is limited.
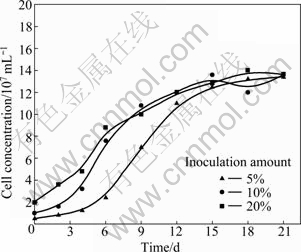
Fig. 3 Bacteria growth curves in different inoculation amounts during bioleaching
Figure 4 shows the copper ion content and redox potential as function of time with different inoculation amounts of L. ferriphilum. It can be seen that the copper ion contents are almost equal under these three different initial cell concentrations at the end of bioleaching. Hence, the leaching efficiency is not determined by the initial inoculation amounts of bacteria. The strains used in biological metallurgy industry are influenced by the environment like temperature, acidity, nutrients, medium (energy), dissolved metal ions and surfactant [12]. Therefore, in the same leaching condition, the number of bacteria is almost the same after a period and barely has influence on bioleaching, which is consistent with the bacteria growth curves above.
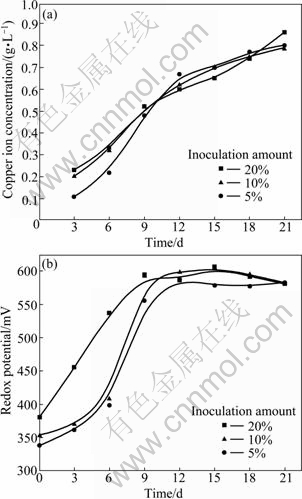
Fig. 4 Copper ion concentration and redox potential as function of time in different inoculation amounts: (a) Copper ion concentration; (b) Redox potential
At the initial stage of bioleaching, the more the total inoculation amount is, the higher the redox potential is. The increasing number of bacteria accelerates the bioleaching rate. But after about 10 d, redox potentials remain stable around 580 mV. The difference of initial cell concentration has no effect on the redox potential, which agrees fairly well with the conclusion above.
3.3 Effect of initial pH value
Copper ion concentration and redox potential as function of time at various initial pH values are shown in Fig. 5. The inoculation amount is 10% and pulp content is 1%. The pH value is not adjusted in the process of bioleaching.
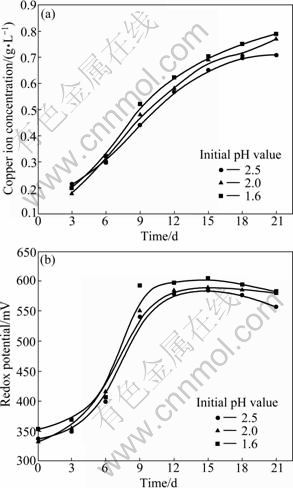
Fig. 5 Copper ion concentration and redox potential as function of time at various initial pH values: (a) Copper ion concentration; (b) Redox potential
When pH range is 1.6-2.5, it could be noticed that bioleaching is not determined by initial pH value. L. ferriphilum activity is not affected. The behavior of redox potential is similar to the changes of copper ion concentration in solution, which indicates that Fe3+ ions play an important role in dissolution of chalcopyrite. Hence, the indirect action is important for the bioleaching of chalcopyrite.
3.4 Effect of external addition of Fe3+
The effects of external addition of Fe3+ on the leaching process and the redox potential are shown in Fig. 6. The pulp content is 1% and the inoculation amount is 10%.
External addition of Fe3+ is beneficial to leaching chalcopyrite. It has been reported that chalcopyrite dissolution rate is strongly affected by ferric concentration, but only at low concentrations [13-14]. Many researchers [13-15] pointed out that chalcopyrite dissolution was improved when the concentration of ferric sulphate was increased from 0.001 to 0.1 mol/L, because Fe3+ is responsible for chalcopyrite dissolution. The external addition of Fe3+ can produce a high initial redox potential but cannot maintain it. At the initial stage of leaching process, the redox potential decreases (reaction (2)); after 3 d, the redox potential begins to rise (reaction (1)). This explains that high concentration ferric ion can promote the bioleaching of chalcopyrite.
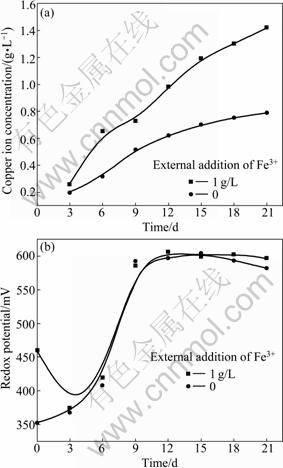
Fig. 6 Copper ion concentration of chalcopyrite and redox potential as function of time in solution with external addition of Fe3+: (a) Copper ion concentration; (b) Redox potential
The changes of pH values during bioleaching are presented in Fig. 7. It is obvious that pH ascends quickly with additional ferric ions, because the strong oxidizing Fe3+ can accelerate chalcopyrite dissolution (reaction (2)). During bioleaching, a large amount of ferric ions generate in solution which promote the formation of jarosite (reaction (3)), which is an acid production process.
3.5 Microstructure of bioleaching residues
From bioleaching tests mentioned above, it can be noticed that dissolution of chalcopyrite is not complete, and copper ion concentration is very low during bioleaching. The low leaching rate may have a great relationship with passivation layer formed during leaching, which can hinder the bioleaching of chalcopyrite [9-10, 16-20].
In Fig. 8, X-ray diffraction pattern of residues bioleached by L. ferriphilum for 21 d shows that sulfur and jarosite are present as secondary phases. This proves that sulfur and jarosite precipitations occur during the leaching process. The SEM image shows that residues leached by L. ferriphilum are covered with oxidation products. A layer is present on the surface of chalcopyrite. This layer would inhibit the diffusion of microorganisms, nutrients and reaction products to and from the mineral surface.
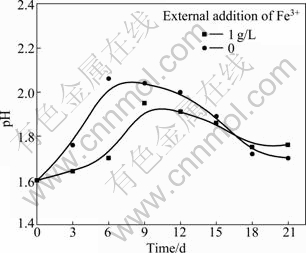
Fig. 7 pH value as function of time in different amounts of external addition of Fe3+
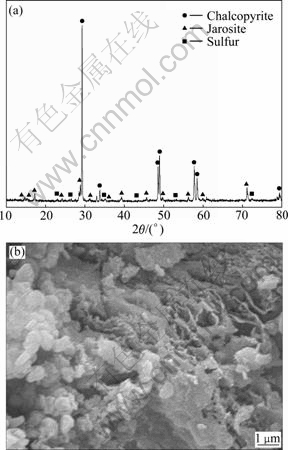
Fig. 8 XRD pattern (a) and SEM image (b) of leached chalcopyrite residues by L. ferriphilum with pulp concentration of 3%
Table 1 lists the results of EDS. Before leaching, the mass fractions of elements in chalcopyrite for Cu, Fe and S are 30.74%, 30.64% and 33.24%, respectively. The molar ratio of Cu, Fe to S is 1:1.14:2.16, which is very close to the theoretical molar ratio of chalcopyrite. In residues leached by L. ferriphilum, the molar ratio is 1: 3.48:4.18. The results indicate that the surface sulphur and iron element are relatively rich after bioleaching of chalcopyrite due to the formation of sulfur and jarosite, which is consistent with the leaching experiments and XRD results.
Table 1 Results of EDS for residues by L. ferriphilum and mixed bacteria

Many researchers [10, 16-19] report that jarosite is responsible for chalcopyrite passivation, especially when the redox potential is high. Some other researchers [9, 20] believe that the formation of sulfur is a main reason for the declining of bioleaching rate. In this experiment, a tight passivation layer mainly containing jarosite and sulfur has formed on the mineral surface, and this layer has blocked the continuous copper extraction during bioleaching.
4 Conclusions
1) The pulp content has an obvious effect on chalcopyrite bioleaching by L. ferriphilum. The leaching speed is faster at low pulp content than that at high pulp content. However, the various copper ion concentrations are not determined by the different inoculations of bacteria. In addition, pH values ranging from 1.6 to 2.5 have a little effect on the leaching rate.
2) Redox potential has great effect on the leaching rate of chalcopyrite. Certain value of redox potential is beneficial to the leaching of chalcopyrite. Fe3+ ions play an important role in the bioleaching of chalcopyrite.
3) A tight passivation layer formed on the surface can cause a decrease of chalcopyrite dissolution. The components of the passivation layer are mainly jarosite and sulfur.
References
[1] Pradhan N, Nathasharma K C, Rao K S, Sukla L B, Mishra B K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering, 2008, 21(5): 355-365.
[2] Qiu Mu-qing, Xiong Shui-ying, Zhang Wei-min. Efficacy of chalcopyrite bioleaching using a pure and a mixed bacterium [J]. Journal of University of Science and Technology Beijing, 2006, 13(1): 7-10.
[3] WU Xue-liang, DING Jian-nan, GAO Jian, LIU Xin-xing, QIU Guan-zhou. Isolation and identification of metal-resistant iron- oxidizing bacteria [J]. Minerals and Metallurgical Processing, 2007, 24(1): 57-60.
[4] ZENG Wei-ming, QIU Guan-zhou, ZHOU Hong-bo, PENG Juan-hua, CHEN Miao, TAN S N, CHAO Wei-liang, LIU Xue-duan, ZHANG Yan-sheng. Community structure and dynamics of the free and attached microorganisms during moderately thermophilic bioleaching of chalcopyrite concentrate [J]. Bioresource Technol, 2010, 101(18): 7068-7075.
[5] Antonio G M, Elena G T, Mercedes M P. Evaluation of Leptospirillum spp. in the Rio Tinto, a model of interest to biohydrometallurgy [J]. Hydrometallurgy, 2008, 94: 155-161.
[6] Baker B J, Banfield J E. Microbial communities in acid mine drainage [J]. Microbiology Ecology, 2003, 44(2): 139-152.
[7] GU Guo-hua, SU Li-jun, CHEN Ming-lian, SUN Xiao-jun, ZHOU Hong-bo. Bio-leaching effects of Leptospirillum ferriphilum on the surface chemical properties of pyrite [J]. Mining Science and Technology, 2010, 20(2): 286-291. (in Chinese)
[8] SOKIC M D, MATKOVIC V L, MARKOVIC B R, STRBAC N D, ZIVKOVIC D T. Passivation of chalcopyrite during the leaching with sulphuric acid solution in presence of sodium nitrate [J]. Hem Ind, 2010, 64(4): 343-350.
[9] KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. Int J Miner Process, 2008, 86(1/2/3/4): 1-17.
[10] Stott M B, Watling H R, Franzmann P D, Sutton D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching [J]. Minerals Engineering, 2000, 13(10-1): 1117-1127.
[11] Watling H R. The bioleaching of sulphide minerals with emphasis on copper sulphides-A review [J]. Hydrometallurgy, 2006 84: 81-108.
[12] Yao Guo-cheng, Ruan Reng-man, Wen Jian-kang. Ore leaching bacterin commonly used in biological metallurgy and basic methods of their culture improvement [J]. Metal Mine, 2002, 317: 26-29.
[13] Kametani H, Aoki A. Effect of suspension potential on the oxidation rate of copper concentrate in a sulfuric acid solution [J]. Metallurgical and Materials Transactions B, 1985, 16B: 695-705.
[14] Hirato T, Majima H, Awakura Y. The leaching of chalcopyrite with ferric sulfate [J]. Metallurgical and Materials Transactions B, 18B: 489-496.
[15] Córdobaa E M, Mu?ozb J A, Blázquezb M L, Gonzálezb F, Ballester A. Leaching of chalcopyrite with ferric ion. Part I: General aspects [J]. Hydrometallurgy 2008, 93: 81-87.
[16] ?ke S, Andrei S, Jan P. XPS characterization of chalcopyrite chemically and bio-leached at high and low redox potential [J]. Minerals Engineering, 2005, 18: 505-515.
[17] GHAHREMANINZHAD A, ASSELIN E, DIXON D G. Electrochemical evaluation of the surface of chalcopyrite during dissolution in sulfuric acid solution [J]. Electrochim Acta, 2010, 55(18): 5041-5056.
[18] BALLESTER A, CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F. Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 degrees C [J]. Miner Eng, 2009, 22(3): 229-235.
[19] YU Run-lan, TAN Jian-xi, GU Gou-hua, HU Yue-hua, QIU Guan- zhou. Mechanism of bioleaching chalcopyrite by Acidithiobacillus ferrooxidans in agar-simulated extracellular polymeric substances media [J]. Journal of Central South University of Technology, 2010, 17(1): 56-61.
[20] Bevilaqua D, Diéz-Perezb I, Fugivarac C S, Sanzb F, Benedettic A V, Garcia Jr O. Oxidative dissolution of chalcopyrite by Acidithiobacillus ferrooxidans analyzed by elect rochemical impedance spectroscopy and atomic force microscopy [J]. Bioelectrochemistry, 2004, 64: 79-84.
(Edited by HE Yun-bin)
Foundation item: Project(2010CB630903) supported by the National Basic Research Program of China
Received date: 2011-03-23; Accepted date: 2011-05-16
Corresponding author: GU Guo-hua, Professor, PhD; Tel: +86-731-88830545; E-mail: guguohua@126.com