间隙大小对瞬时液相连接IN-738LC高温合金显微组织的影响
来源期刊:中国有色金属学报(英文版)2016年第2期
论文作者:Vahid MALEKI Hamid OMIDVAR Mohammad-reza RAHIMIPOUR
文章页码:437 - 447
关键词:IN-738LC 高温合金;瞬时液相连接;间隙大小;完全等温凝固
Key words:IN-738LC superalloy; transient liquid phase (TLP) bonding; gap size; complete isothermal solidification
摘 要:以AMS 4777粉末为填料,研究瞬时液相连接IN-738LC高温合金的显微组织演变,获得完全等温凝固时间。研究间隙大小和连接时间对接头组织的影响。当间隙大小为40 μm时,完全等温凝固时间为45 min。在不完全等温凝固的情况下,连接区的残余液相在非平衡条件下冷却,形成γ-γ共晶相。间隙大小与时间存在非线性关系。随着间隙增大,共晶相变宽。在扩散影响区,发现大量合金元素,其浓度达到峰值,这是由于形成了硼化物和硅化物等金属间化合物。随着间隙增加,所需连接时间增加,合金元素有更多的时间进行扩散和分布至更大区域。因此,合金元素浓度随间隙增加缓慢降低。已有双相模型不能准确预测IN-738LC-AMS 4777-IN738LC瞬时液相连接系统的完全等温凝固时间。
Abstract: In order to investigate the microstructure evolution and gain complete isothermal solidification time, transient liquid phase (TLP) bonding of IN-738LC superalloy was carried out using powdered AMS 4777 as the filler metal. The influence of gap size and bonding time on the joints was investigated. For example, complete isothermal solidification time for 40 μm gap size was obtained as 45 min. In the case of lack of completion of isothermal solidification step, the remained molten interlayer cooled in the bonding zone under non-equilibrium condition and γ–γ′ eutectic phase formed in that area. The relationship between gap size and holding time was not linear. With the increase in gap size, eutectic phase width became thicker. In the diffusion affected zone, a much larger amount of alloying elements were observed reaching a peak. These peaks might be due to the formation of boride or silicide intermetallic. With the increase in gap size, the time required for bonding will increase, so the alloying elements have more time for diffusion and distribution in farther areas. As a result, concentrations of alloying elements decreased slightly with the increase in the gap size. The present bi-phasic model did not properly predict the complete isothermal solidification time for IN-738LC-AMS 4777-IN-738LC TLP bonding system.
Trans. Nonferrous Met. Soc. China 26(2016) 437-447
Vahid MALEKI1, Hamid OMIDVAR1, Mohammad-reza RAHIMIPOUR2
1. Department of Mining and Metallurgical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Tehran 15875-4413, Iran;
2. Department of Ceramic, Materials and Energy Research Center, Karaj 31787-316, Iran
Received 30 March 2015; accepted 11 September 2015
Abstract: In order to investigate the microstructure evolution and gain complete isothermal solidification time, transient liquid phase (TLP) bonding of IN-738LC superalloy was carried out using powdered AMS 4777 as the filler metal. The influence of gap size and bonding time on the joints was investigated. For example, complete isothermal solidification time for 40 μm gap size was obtained as 45 min. In the case of lack of completion of isothermal solidification step, the remained molten interlayer cooled in the bonding zone under non-equilibrium condition and γ–γ′ eutectic phase formed in that area. The relationship between gap size and holding time was not linear. With the increase in gap size, eutectic phase width became thicker. In the diffusion affected zone, a much larger amount of alloying elements were observed reaching a peak. These peaks might be due to the formation of boride or silicide intermetallic. With the increase in gap size, the time required for bonding will increase, so the alloying elements have more time for diffusion and distribution in farther areas. As a result, concentrations of alloying elements decreased slightly with the increase in the gap size. The present bi-phasic model did not properly predict the complete isothermal solidification time for IN-738LC-AMS 4777-IN-738LC TLP bonding system.
Key words: IN-738LC superalloy; transient liquid phase (TLP) bonding; gap size; complete isothermal solidification
1 Introduction
JALILVAND et al [1] reported that IN-738LC is a polycrystalline nickel-based superalloy with superior mechanical properties at elevated temperatures and the exceptional high-temperature strength of IN-738LC is related to the presence of Ni3(Al, Ti) γ′ intermetallic FCC phase in the γ solid solution matrix. POURANVARI et al [2] and ABDELFATAH and OJO [3] expressed that the strengthened nickel-based superalloys are extensively used in the hot sections of aero-engine and power generation turbines. In addition, they offer excellent high-temperature tensile strength, stress rupture and creep properties, fatigue strength, thermo-mechanical fatigue, oxidation and corrosion resistance, and microstructural stability at elevated temperatures. JALILVAND et al [4] reported that for repairing or joining purposes, fusion welding, diffusion bonding, and brazing are the three main techniques which are commonly used in the industry. SHENG et al [5] expressed that transient liquid phase (TLP) bonding is used more widely in bonding alloys which are sensitive to crack during fusion welding such as superalloys. GRANT et al [6] reported that the resulted bonds have a higher melting point than the bonding temperature. So, POURANVARI et al [2,7] believe that TLP bonding is considered as a preferred repairing or joining process for nickel-based superalloys due to its ability to produce near-ideal bonds. Also, LEE et al [8] reported that TLP bonding has been applied to many cast superalloys. LIU et al [9] reported that in TLP bonding, a filler alloy with chemical composition close to the base alloy and a melting point which is slightly less than it is often preplaced between the two surfaces to be joined and then the entire assembly is heated to the bonding temperature. LEE et al [8] expressed that a liquid film is temporarily formed at the bonding temperature during TLP bonding and is isothermally solidified by the diffusion of melting point depressive (MPD) elements such as boron and silicon into the parent metal, consequently, excellent bond quality can be expected. However, POURANVARI et al [10] believe that these elements are incorporated into intermetallic phases such as borides, silicides, and phosphides during athermal eutectic-type solidification of the liquid phase during cooling stage of the TLP process. In general, CAO et al [11] expressed that there are three distinct stages for TLP bonding: 1) interlayer melting and base metal dissolution, 2) isothermal solidification, and 3) solid-state homogenization. YANG et al [12] believe that brazing, multiple intermetallic phases in the bonding region lead to the bond brittleness and susceptibility to mechanical decline. Although the TLP bonding uses the isothermal solidification to prevent the formation of brittle phases in the bonding region, it is limited to a small gap which does not exceed 250 μm. If sufficient time for complete isothermal solidification is not allowed at the bonding temperature, GHONEIM and OJO [13] reported that formation of eutectic micro- constituents could occur along the centerline of the bond. Appropriate holding time required for complete isothermal solidification should be determined to obtain the proper microstructure in the centerline. POURANVARI [14] and BAKHTIARI and EKRAMI [15] indicated that at insufficient holding time, athermal solidification of the residual liquid phase may lead to eutectic intermetallic formation, which in turn degrades the mechanical strength, service temperature, and corrosion resistance of the bonds compared with the base metal. Therefore, GHONEIM and OJO [13] proposed that the key benefit of the TLP bonding relies on the fact that, through proper process optimization, it is possible to prevent the formation of the deleterious eutectic microconstituent that decreases the properties of materials joined by conventional brazing techniques.
solid solution matrix. POURANVARI et al [2] and ABDELFATAH and OJO [3] expressed that the strengthened nickel-based superalloys are extensively used in the hot sections of aero-engine and power generation turbines. In addition, they offer excellent high-temperature tensile strength, stress rupture and creep properties, fatigue strength, thermo-mechanical fatigue, oxidation and corrosion resistance, and microstructural stability at elevated temperatures. JALILVAND et al [4] reported that for repairing or joining purposes, fusion welding, diffusion bonding, and brazing are the three main techniques which are commonly used in the industry. SHENG et al [5] expressed that transient liquid phase (TLP) bonding is used more widely in bonding alloys which are sensitive to crack during fusion welding such as superalloys. GRANT et al [6] reported that the resulted bonds have a higher melting point than the bonding temperature. So, POURANVARI et al [2,7] believe that TLP bonding is considered as a preferred repairing or joining process for nickel-based superalloys due to its ability to produce near-ideal bonds. Also, LEE et al [8] reported that TLP bonding has been applied to many cast superalloys. LIU et al [9] reported that in TLP bonding, a filler alloy with chemical composition close to the base alloy and a melting point which is slightly less than it is often preplaced between the two surfaces to be joined and then the entire assembly is heated to the bonding temperature. LEE et al [8] expressed that a liquid film is temporarily formed at the bonding temperature during TLP bonding and is isothermally solidified by the diffusion of melting point depressive (MPD) elements such as boron and silicon into the parent metal, consequently, excellent bond quality can be expected. However, POURANVARI et al [10] believe that these elements are incorporated into intermetallic phases such as borides, silicides, and phosphides during athermal eutectic-type solidification of the liquid phase during cooling stage of the TLP process. In general, CAO et al [11] expressed that there are three distinct stages for TLP bonding: 1) interlayer melting and base metal dissolution, 2) isothermal solidification, and 3) solid-state homogenization. YANG et al [12] believe that brazing, multiple intermetallic phases in the bonding region lead to the bond brittleness and susceptibility to mechanical decline. Although the TLP bonding uses the isothermal solidification to prevent the formation of brittle phases in the bonding region, it is limited to a small gap which does not exceed 250 μm. If sufficient time for complete isothermal solidification is not allowed at the bonding temperature, GHONEIM and OJO [13] reported that formation of eutectic micro- constituents could occur along the centerline of the bond. Appropriate holding time required for complete isothermal solidification should be determined to obtain the proper microstructure in the centerline. POURANVARI [14] and BAKHTIARI and EKRAMI [15] indicated that at insufficient holding time, athermal solidification of the residual liquid phase may lead to eutectic intermetallic formation, which in turn degrades the mechanical strength, service temperature, and corrosion resistance of the bonds compared with the base metal. Therefore, GHONEIM and OJO [13] proposed that the key benefit of the TLP bonding relies on the fact that, through proper process optimization, it is possible to prevent the formation of the deleterious eutectic microconstituent that decreases the properties of materials joined by conventional brazing techniques.
This work aimed to investigate the correlation between the gap size and microstructure during TLP bonding process of cast IN-738LC using Ni-Cr-Si-B-Fe (AMS 4777) filler metal. In addition, isothermal solidification completion time for different gap sizes was obtained.
2 Experimental
2.1 Materials
Chemical composition of the base metal, cast IN-738LC superalloy obtained by induced coupled plasma (ICP) spectroscopy is given in Table 1. At first, the primary specimens with the sizes of 100 mm ×10 mm × 5 mm (Fig. 1(a)) were cut from the as-received base superalloy using a wire electro-discharge machine.
Table 1 Chemical composition of base alloy obtained by wet chemistry (ICP) analysis (mass fraction, %)
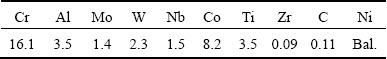
TLP bonding was carried out using powdered AMS 4777 as the filler metal alloy with the chemical composition given in Table 2 for joining specimens. Then, the bonded samples with the sizes of 10 mm × 10 mm × 10 mm were cut from it.
Table 2 Specifications of AMS 4777 filler metal [1,4]

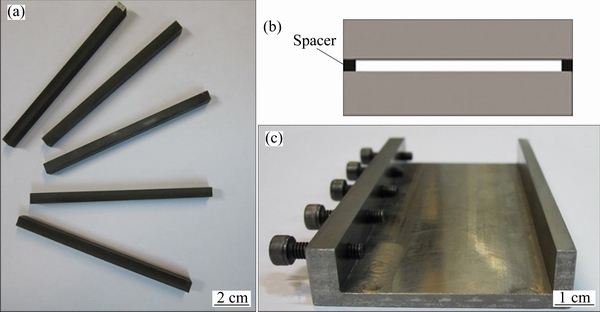
Fig. 1 Primary specimens (a), schematic diagram of spacers between two base metal specimens (b) and fixture to prevent movement of samples (c)
2.2 Experimental techniques
In order to remove the surface oxide layer formed during electro-discharge machine cutting, contacting surface of the samples was polished by emery until grade 1000. Then, the specimens were ultrasonically cleaned and stored in acetone for 30 min. Fixed gap size (40, 80, 120, 160, and 200 μm) samples were produced using the combination of 40 and 60 μm stainless steel spacers between the two base metal surfaces (Fig. 1(b)). For example, to create the 160 μm gap size, two 60 μm and one 40 μm spacers were used between the two base metal surfaces. A stainless steel fixture was used to prevent the movement of the samples during the TLP process (Fig. 1(c)). Bonding process for all samples was carried out in a tubular vacuum furnace with the heating rate of 15 °C/min under the vacuum of approximately 5.33×10-3 Pa at the bonding temperature of 1120 °C and different bonding time (30, 45, 60, 80, 105, and 120 min). All the samples were furnace cooled to the room temperature once the holding time was completed. TLP bonded samples were sectioned perpendicular to the bonding zone. In order to show the bonded zone microstructure, the prepared samples were etched by Kalling’s reagent. Also, molybdenum-acid etchant which etches preferentially the γ′ phase was used to indicate the γ–γ′ microstructure of the bonds (Table 3).
Table 3 Specifications of etchant reagents

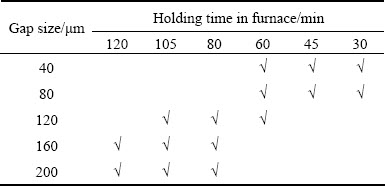
Optical microscopy (OM) and scanning electron microscopy (SEM) were used to study the microstructures of the bonds. In order to obtain the compositional analysis of the bonds and SEM images, MIRA TESCAN scanning electron microscope equipped with an energy disperse X-ray spectrometer (EDS) system and CAMBRIDGE 360 scanning electron microscope were employed. Each gap size was bonded at three different time to obtain the optimized complete isothermal solidification time (Table 4).
Table 4 Characterization of joining
3 Results and discussion
3.1 Material’s microstructure
ZHANG et al [16] reported that in pure nickel metal ingot, grain boundaries are flat; but, in the as-received IN-738LC, grain boundaries are not flat, even are serrated (Fig. 2(a)). DANFLOU et al [17], CHANG et al [18] and CARTER et al [19] believe that the grain boundary serrations are correlated with the precipitation of adjacent coarse γ′ precipitates along the grain boundaries with the non-uniform movement of grain boundaries. Also, BEDDOES and WALLACE [20] reported that the grain boundary serration in IN-738LC superalloy had a significant potential for improving high-temperature mechanical properties by preventing the slide of grain boundaries. Optical microscopy examination of the as-received IN-738LC showed the dendritic structure of the γ context (Fig. 2(b)). This phase had FCC crystal lattice and contained intergranular and grain boundary non-uniform carbide precipitation; thus, the most abundant carbide precipitation is mainly M23C6.
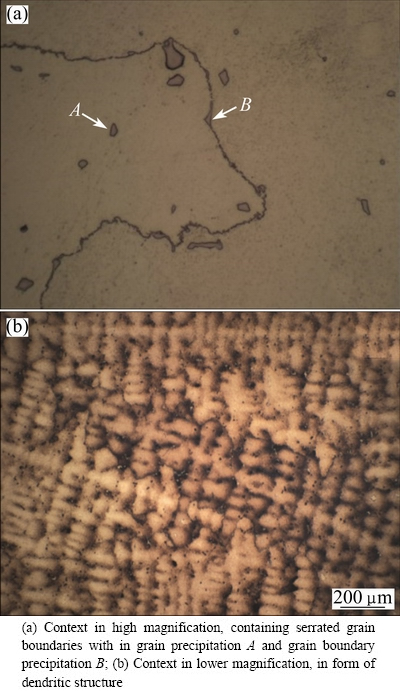
Fig. 2 Optical micrographs of as-received IN-738LC
High magnification SEM image of as-received alloy showed a context containing precipitation particles γ′ (Fig. 3(a)). Figure 3(b) shows SEM image distribution of γ–γ′ non-uniform eutectic islands and blocky carbides in the context. JALILVAND et al [4] expressed that the formation of the non-equilibrium γ–γ′ eutectic was the result of microsegregation during the ingot solidification and also dendritic micro segregation of γ′ particles occurred during the casting and solidification processes resulted in different sizes of γ′ particles.
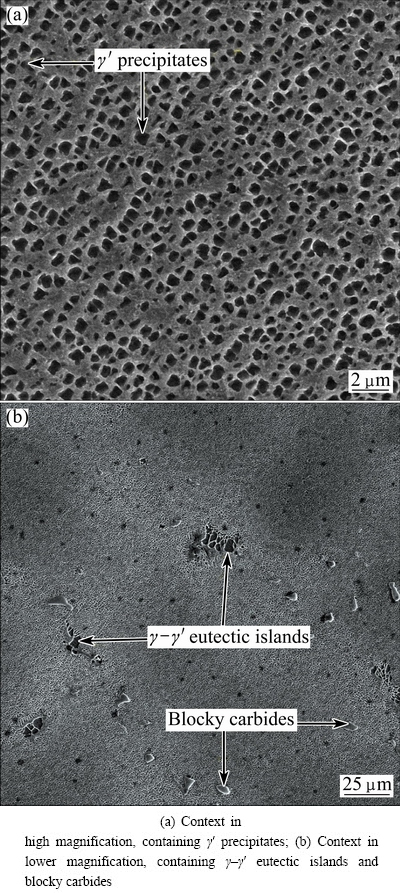
Fig. 3 SEM images of as-received IN-738LC
DONACHIE and DONACHIE STEPHEN [21] expressed that topologically close-packed phases (TCP), such as μ and σ in superalloys, may not often be present initially in the as-processed microstructure, but appear after long-time exposure.
3.2 TLP microstructure
During the TLP bonding process, the insert filler alloy melts or reacts with the base metal substrate and causes the formation of a liquid phase. Then, the melting point of the liquated phase is increased by the diffusion of alloying elements into the base metal, which leads to the start of the isothermal solidification at the liquid/solid interface. With the progress of isothermal solidification, the volume of liquid phase is decreased and the liquid /solid interface regresses beyond the bond centerline. Since there is no solute rejection at the liquid/solid interface during the isothermal solidification, an ideal bond appears, which only contains nickel-rich γ solid solution. This phase has been a desirable phase in TLP bonding IN-738LC superalloy and prevents from the formation of the secondary phase. Insufficient holding time for the completion of isothermal solidification would result in the transformation of residual liquid into athermal solidified phases, mainly consisting of intermetallic and eutectic compounds in the bonding zone.
Figure 4 presents SEM image of different zones of the athermal solidified bond made with 80 μm gap size at 45 min of holding time observed in the secondary electron mode. According to this figure, after bonding, the sample was composed of four distinct zones: 1) Athermally solidified zone (ASZ); 2) Isothermally solidified zone (ISZ); 3) Diffusion affected zone (DAZ); and 4) Base material (BM). According to previous works in which MOSALLAEE et al [22] and JALILVAND et al [23] had been investigated, microstructure of the ASZ is composed of Ni-rich boride, Cr-rich boride, and Ni-based γ solid solution phases, formed by eutectic-type transformation. The microstructure of the ISZ often consists of Ni solid solution.

Fig. 4 SEM image of different zones of athermal solidified bond, made with 80 μm gap size and 45 min of holding time
Figure 5 presents Widmanstatten morphology of the secondary intermetallic particles in the DAZ for the 80 μm gap size sample prepared for 60 min holding time. POURANVARI et al [24] indicated Widmanstatten morphology of the DAZ in TLP bonding of the GTD-111 nickel-based superalloy. JALILVAND et al [1] reported that the microstructure of the DAZ consists of extensive blocky and acicular B-rich intermetallic particles. Formation of the intermetallic particles in the DAZ can be due to the diffusion of boron from the interlayer into the base alloy during the solution and isothermal solidification processes.

Fig. 5 DAZ of complete isothermal solidified bond, made with 80 μm gap size and 60 min of holding time
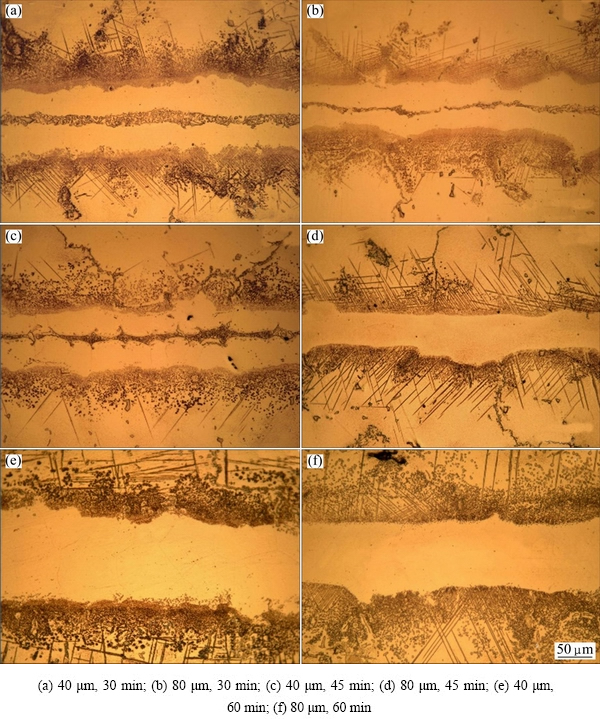
Fig. 6 Optical images of TLP bonded
3.3 Effect of gap size on bond microstructure
In order to examine the effect of gap size on the microstructure of TLP-bonded joints, according to Table 4, various gaps (40, 80, 120, 160, and 200 μm) assembly of IN-738LC base metal were bonded using AMS 4777 (AWS BNi-2) filler metal at 1120 °C for different bonding time (30, 45, 60, 80, 105, and 120 min). Microstructure of the samples at two different gap sizes, obtained by OM, is illustrated in Fig. 6. JALILVAND et al [4] reported that, with increased penetration of MPD (B and Si), the elements from interlayer to the base metal during the solidification of the interlayer increased as well. According Figs. 6(a) and (b) at the same time for two different gap sizes, thickness of the centerline eutectic in Fig. 6(b) was observed to form across the bond layer, which became wider, denser, and continuous with further increase in the gap size, because penetration distance of MPD elements was increased and consequently diffusion rate was decreased. Thus, a more amount of interlayer had lower solidification temperature. Such lower solidification temperature stopped the bond from having isothermal solidification. As a result, at the bonding temperature, the liquid phase would be ready for eutectic reaction. So, for a desirable bond, it is suitable for all the interlayers to be solidified at bonding temperature. JALILVAND et al [4] indicated that isothermal solidification during TLP bonding greatly depends on the diffusion of MPD elements from the liquid interlayer into the base metal. The rate of isothermal solidification at a constant bonding temperature and time was expected to be the same in different gap size joints. So, the volume of the residual liquid present after 30 min holding time should increase with the increase in gap size; as a result, more residual liquid phase would be available for the eutectic reaction during cooling, which would explain the observed increase in the width of the centerline eutectic with increased gap size at constant bonding temperature and time.
According to Figs. 6(b) and (d) at the same gap size, thickness of the centerline eutectic phase was observed to form across the bond layer, which became narrower with further increase in holding time in Fig. 6(d), because increase in holding time caused an increase in the diffusion rate of MPD elements from the liquid interlayer into the base metal and decrease solidification temperature of the interlayer. The observations (Fig. 6(c)) showed that increase in bonding time to 45 min for 40 μm gap size resulted in a eutectic-free bond; so, isothermal solidification was completed during this length of bonding time. According to Figs. 6(c) and (e), joints in complete isothermal were solidified at the same gap size; with further increase in holding time, the microstructure became more uniform in Fig. 6(e), because it was more homogenized. Difference between these two complete isothermal solidified joints was related to homogenization rate. Also, Fig. 6(f) shows that sufficient bonding time was 60 min for obtaining an eutectic-free bond with 80 μm gap size. Complete isothermal solidified bond for 120 μm gap size was obtained at 105 min (Fig. 7(c)).
Complete isothermal solidification bond was not obtained for 160 and 200 μm gap sizes, even in the samples prepared for 120 min holding time (Fig. 8). Centerline eutectic phase in the 200 μm gap size sample was denser and more continuous than that in the 160 μm gap size sample, because with increased diffusion distance (Fig. 8(b)), in large gap sizes, complete isothermal solidification time would be very long.
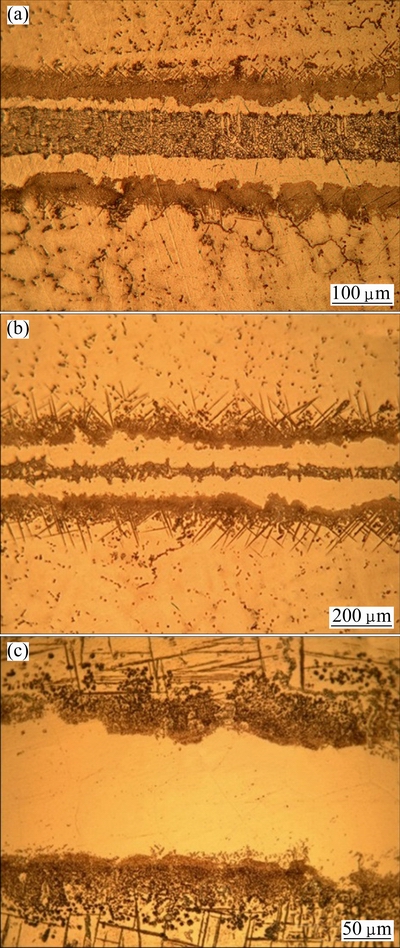
Fig. 7 Optical images of TLP bonded 120 μm joint with 60 min (a), 80 min (b) and 105 min (c) holding time
3.4 Effect of gap size on complete isothermal solidification time
Figure 9 shows the plot of complete isothermal solidification time against gap size for three different gap sizes. JALILVAND et al [4] investigated on TLP bonding, and showed that the distance of the penetration of the diffusing elements from interlayer into the base alloy was expected to be commensurate to the square root of bonding time; thereby, the plot will not be linear. As seen in Fig. 9, with increase in gap size from 40 to 80 μm, complete isothermal solidification time increased by 15 min; but, with increased gap size from 80 to 120 μm, it was increased by 45 min, which could explain the non-linear proportion between gap size and complete isothermal solidification time.
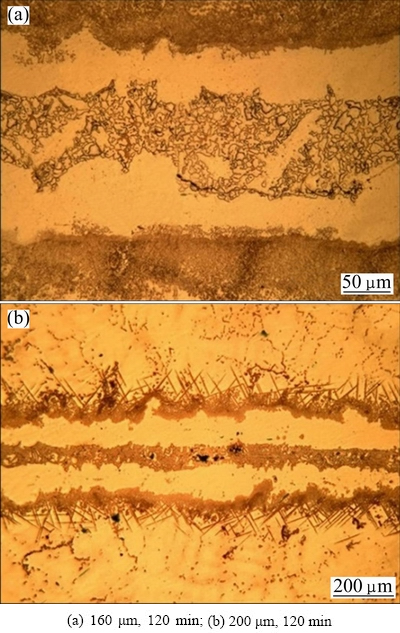
Fig. 8 Optical images of TLP bonded

Fig. 9 Plot of complete isothermal solidification time against gap size
3.5 Effect of gap size on average eutectic width
JALILVAND et al [4] showed that more migration of the liquid/solid interface to the centerline follows a square root correlation with bonding time. Figure 10 shows the plot of average eutectic width versus the square root of holding time in the prepared joints: 80 μm gap size, 60 min (complete isothermal solidified) and 200 μm gap size, 120 min (athermal solidified). According to this figure, increase in holding time caused a decline in average eutectic width because of more diffusion of MPD elements from interlayer into the base metal. JALILVAND et al [4] reported that increase in holding time and diffusion of boron into the base metal resulted in a decline in the centerline eutectic width.
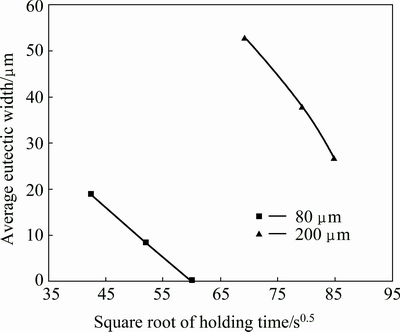
Fig. 10 Plot of average eutectic width versus square root of holding time in prepared joints, 80 μm gap size, 60 min (complete isothermal solidified) and 200 μm gap size, 120 min (athermal solidified)
3.6 Effect of gap size on distribution of alloying elements
Line scan analysis carried out across isothermally solidified joints with 40, 80, and 120 μm gap sizes is shown in Fig. 11. According to this analysis, alloying elements of the base metal which did not exist in the initial filler alloy composition can be considerably seen in ISZ. Indeed, through the alloying elements, only Cr existed in the filler alloy composition. Therefore, the enrichment of TLP bonded joints with base metal alloying elements was due to the base metal dissolution and/or interdiffusion between two intimate solid and liquid phases. In the DAZ, a much larger amount of alloying elements was often observed reaching a peak. These peaks might be due to the formation of boride or silicide intermetallic. With the increase in gap size, the required time for bonding will increase, so the alloying elements have more time for diffusion and distribution in farther areas. As a result, concentrations of alloying elements decreased slightly with the increase in the gap size. The rate at which interdiffusion occurred at different gap sizes was the same. Moreover, dissolution was not reported to change with the width of the bonding zone. Although increased gap size during TLP bonding process increased the amount of dissolved MPD elements in DAZ, it would decrease the concentrations gradient of these elements. Therefore, lower concentrations of these elements can be expected at larger gap sizes. ABDELFATAH and OJO [3] investigated the relationship between solid/liquid interface displacement and holding time and showed that reduction in the solute concentration gradient leads to a deviation from the parabolic relationship between solid/liquid interface displacement and holding time.
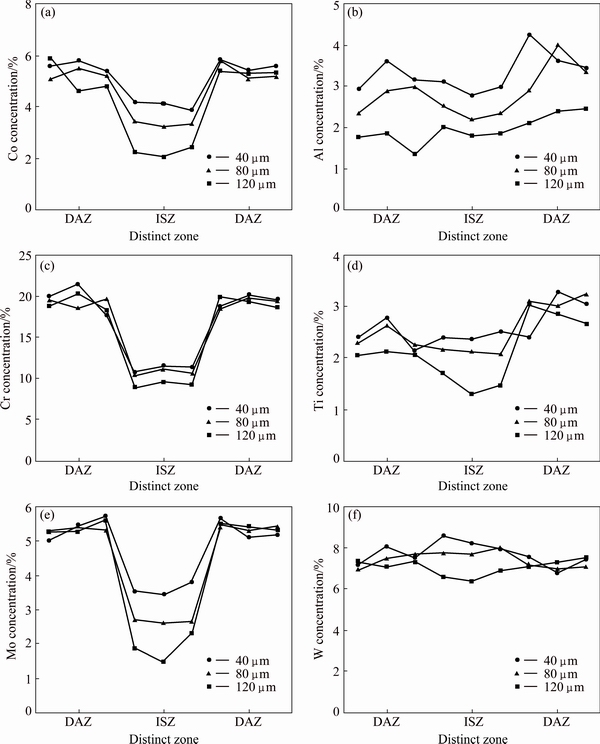
Fig. 11 EDS line scan of isothermally solidified TLP samples at different gap sizes
3.7 Predicting complete isothermal solidification time by analytical model
ARAFIN et al [25] reported that isothermal solidification is the most important stage in TLP bonding process. The analytical model does not consider the real behavior which occurs during TLP bonding process. For example, ZHOU et al [26] reported that the complete isothermal solidification time required for solute homogenization will depend on the solute distribution instantly following the completion of the isothermal solidification stage. PADRONA et al [27] used the bi-phase model considering the problem as a half semi-infinite base metal with a stationary interface and solute concentration is maintained at cs. According to this model, complete isothermal solidification time (tCIS) is forecasted by
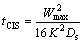 (1)
(1)
where Ds is the diffusion coefficient of the MPD element (here boron) from interlayer to the base metal, Wmax is maximum thickness of the molten interlayer at TLP bonding, and K is a dimensionless parameter which is calculated by the following equation [28]:
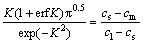 (2)
(2)
where cs and cl are the equilibrium concentrations of the MPD element in the solid and liquid phases at the moving solid–liquid interface at mole fraction, respectively, cm is the initial concentration of the MPD element in the base metal at mole fraction, and Wmax is obtained under the condition of no diffusion in the solid:
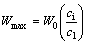 (3)
(3)
where W0 is initial gap size and ci is initial concentration of the MPD element in the filler metal. To investigate the correlation between the results of experiments and analytical model for tCIS, Eq. (2) was numerically solved to obtain the K parameter. The maximum width of the molten interlayer (Wmax) for each gap size was calculated by solving Eq. (3) and using the parameters shown in Table 5. K parameter was calculated by Excel and also diffusion coefficient of boron from interlayer into the base metal was calculated as 3.1×10-10 m2/s.
Table 5 Parameters of analytical model
In Fig. 12, square of the calculated tCIS for 40, 80, and 120 μm gap sizes was compared with the experimental results by plotting tCIS versus gap size. In this figure, a linear proportion can be observed between holding time and gap size for the results of analytical model. ZHOU et al [26] obtained a linear proportion between holding time and gap size using analytical model. The analytical model could also estimate tCIS for 40 μm gap size; but, for 80 and 120 μm gap sizes, there was a visible deviation between the model and experimental results; in addition, the analytical model estimated tCIS to be considerably higher than the experimental results. ZHOU et al [26] believed that in calculations, the solute diffusion into the base metal was overlooked and liquid region was considered as a thin boundary layer and a large bulk during TLP bonding. According to analytical models, ABDELFATAH and OJO [3] showed a decrease in solute concentration gradient below the critical value of alloy elements during TLP bonding process. According to the results, the present bi-phasic model did not predict the complete isothermal solidification time for IN-738LC-AMS 4777-IN-738LC TLP bonding system, so cannot be acceptable and relevant.
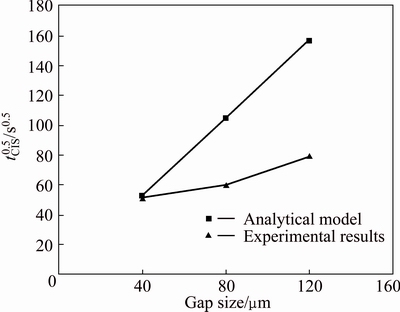
Fig. 12 Comparison of analytical model and experimental results for gap size changes against square of complete isothermal solidification time
BAKHTIARI and EKRAMI [15] showed that at constant bonding temperature and holding time, the amount of MPD elements diffusion from the liquid interlayer into the base metal was expected to be the same for the samples with different gap sizes; so, the width of residual liquid interlayer (eutectic phase width) and complete isothermal solidification time were expected to increase with the increase in gap size. ABDELFATAH and OJO [3] reviewed that analytical TLP bonding models usually assumes base materials as semi-infinite or infinite diffusion media that allow for the constant rate of liquid/solid interface migrations. Figure 13 presents the prediction of liquid/solid interface location from centerline bond against square of holding time according to the analytical model for different gap sizes. Square of complete isothermal solidification time was the intersection place of these lines with horizontal axis.
4 Conclusions
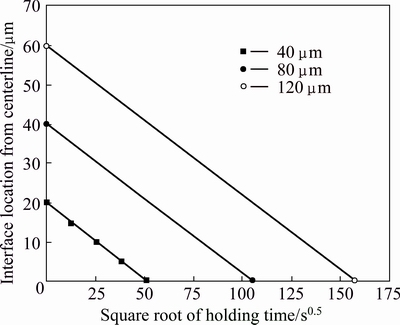
Fig. 13 Prediction of liquid/solid interface location from centerline bond against square of holding time according analytical model for different gap sizes
1) Complete isothermal solidification time for 40, 80, and 120 μm gap sizes were obtained as 45, 60, and 105 min, respectively. Nonetheless, complete isothermal solidification for 160 μm gap size was not complete until 120 min.
2) In the case of the lack of completion of isothermal solidification step, the residual molten interlayer in the bonding zone was cooled down in a non-equilibrium manner in this state and formed γ–γ′ eutectic phase. The eutectic phase substance was the result of athermal solidification and included Ni-rich γ solid solution phase and Ni- and Cr-rich borides. At similar time, with increase in gap size for two bonding gaps with different sizes, width of eutectic phase would be thicker.
3) Due to the diffusion of boron from the interlayer into the base alloy during the TLP bonding process, secondary particles consisting of extensive blocky and acicular B-rich intermetallic with Widmanstatten morphology in the DAZ were developed.
4) In order to achieve isothermally solidified bonds in the experimental work, the proportion between gap size and holding time was not linear.
5) Concentrations of the alloying elements decreased slightly with the increase in the gap size and, so in the DAZ, a rich peak was observed. With increase in gap size, the required time for bonding will increase, so the alloying elements have more time for diffusion and distribution in farther areas. Therefore, lower concentrations of these elements can be expected in larger gap sizes.
6) The bi-phase analytical model only could predict complete isothermal solidification time in 40 μm gap sizes, which was not relevant for IN-738LC-AMS 4777-IN-738LC TLP bonding system in the experimental work.
References
[1] JALILVAND V, OMIDVAR H, SHAKERI H R, RAHIMIPOUR M R. A study on the effect of process parameters on the properties of joint in TLP-bonded inconel 738LC superalloy [J]. Metallurgical and Materials Transactions B, 2013, 44: 1222-1231.
[2] POURANVARI M, EKRAMI A, KOKABI A H. Microstructure– properties relationship of TLP-bonded GTD-111 nickel-base superalloy [J]. Materials Science and Engineering A, 2008, 490: 229-234.
[3] ABDELFATAH M M, OJO O A. On the extension of processing time with increase in temperature during transient-liquid phase bonding [J]. Metall Mater Trans A, 2009, 40: 377-387.
[4] JALILVAND V, OMIDVAR H, SHAKERI H R, RAHIMIPOUR M R. Microstructural evolution during transient liquid phase bonding of Inconel 738LC using AMS 4777 filler alloy [J]. Materials Characterization, 2013, 75: 20-28.
[5] SHENG N, LIU J, JIN T, SUN X, HU Z. Precipitation behaviors in the diffusion affected zone of TLP bonded single crystal superalloy joint [J]. Materials Science, 2015, 31: 129-134.
[6] GRANT O, COOK I I I, CARL D S. Overview of transient liquid phase and partial transient liquid phase bonding [J]. Materials Science, 2011, 46: 5305-5323.
[7] POURANVARI M, EKRAMI A, KOKABI A H. TLP bonding of cast IN718 nickel based superalloy: Process–microstructure–strength characteristics [J]. Materials Science Engineering A, 2013, 568: 76-82.
[8] LEE B K, SONG W Y, KIM D U, WOO I S, KANG C Y. Effect of bonding temperatures on the transient liquid phase bonding of a directionally solidified Ni-based superalloy, GTD-111 [J]. Metals and Materials International, 2007, 13: 59-65.
[9] LIU J D, JIN T, ZHAO N, WANG Z, SUN X, GUAN H, HU Z. Microstructural study of transient liquid phase bonded DD98 and K465 superalloys at high temperature [J]. Materials Characterization, 2011, 62: 545-553.
[10] POURANVARI M, EKRAMI A, KOKABI A H. Transient liquid phase bonding of wrought IN 718 nickel based superalloy using standard heat treatment cycles: Microstructure and mechanical properties [J]. Material and Design, 2013, 50: 694-701.
[11] CAO J, WANG Y F, SONG X G, LI C, FENG J C. Effects of post- weld heat treatment on microstructure and mechanical properties of TLP bonded Inconel 718 superalloy [J]. Materials Science Engineering A, 2014, 590: 1-6.
[12] YANG Y H, XIE Y J, WANG M S, YE W. Microstructure and tensile properties of nickel-based superalloy K417G bonded using transient liquid-phase infiltration [J]. Material and Design, 2013, 51: 141-147.
[13] GHONEIM A, OJO O A. Microstructure and mechanical response of transient liquid phase joint in Haynes 282 superalloy [J]. Materials Characterization, 2011, 62: 1-7.
[14] POURANVARI M. Isothermal solidification during transient liquid-phase bonding of GTD-111/Ni-Si-B/GTD-111 [J]. Materials Processing Technology, 2014, 48: 113-118.
[15] BAKHTIARI R, EKRAMI A. The effect of gap size on the microstructure and mechanical properties of the transient liquid phase bonded FSX-414 superalloy [J]. Material and Design, 2012, 40: 130-137.
[16] ZHANG H, MENDELEV M I, SROLOVITZ D J. Computer simulation of the elastically driven migration of a flat grain boundary [J]. Acta Materialla, 2004, 52: 2569-2576.
[17] DANFLOU H L, MARTY M, WALDER A. Formation of serrated grain boundaries and their effect on the mechanical properties in a P/M nickel base superalloy [C]//Proceedings of the 7th International Symposium on Superalloy. Cedex-France: The Minerals, Metals & Materials Society, 1992: 63-72.
[18] CHANG M, KOUL A K, COOPER C. Damage tolerance of P/M turbine disc materials [C]//Proceedings of the 8th International Symposium on Superalloy. Pennsylvania, Warrendale: The Minerals, Metals & Materials Society: 677-685.
[19] CARTER J L W, ZHOU N, SOSA J M, SHADE P A, PILCHAK A L, KUPER M W, WANG Y, FRASER H L, UCHIC M D, MILLS M J. Characterization of strain accumulations at grain boundaries of nickel based superalloys [C]//Proceedings of the 8th International Symposium on Superalloy. Seven Springs, PA: John Wiley & Sons, 2012: 43-52.
[20] BEDDOES J C, WALLACE W. Heat treatment of hot iso statically processed IN-738 investment castings [J]. Metallography, 1980, 13: 185-194.
[21] DONACHIE M J, DONACHIE STEPHEN J. Superalloys a technical guide [M]. 2th ed. Materials Park, OH: ASM International, 2002.
[22] MOSALLAEE M, EKRAMI A, OHSASA K, MATSUURA K. Microstructural evolution in the transient-liquid-phase bonding area of IN-738LC/BNi-3/IN-738LC [J]. Metallurgical and Material Transaction A, 2008, 39: 2389-2402.
[23] JALILVAND V, OMIDVAR H, RAHIMIPOUR M R, SHAKERI H R. Influence of bonding variables on transient liquid phase bonding behavior of nickel based superalloy IN-738LC [J]. Material and Design, 2013, 52: 36-46.
[24] POURANVARI M, EKRAMI A, KOKABI A H. Microstructure development during transient liquid phase bonding of GTD-111 nickel-based superalloy [J]. Alloys and Compound, 2008, 461: 641-647.
[25] ARAFIN M A, MEDRAJ M, TURNER D P, BOCHER P. Transient liquid phase bonding of Inconel 718 and inconel 625 with BNi-2: Modeling and experimental investigations [J]. Materials Science Engineering A, 2007, 447: 125-133.
[26] ZHOU Y, GALE W F, NORTH T H. Modelling of transient liquid phase bonding [J]. International Materials Reviews, 1995, 40: 181-197.
[27] PADRONA T, KHANA T I, KABIR M J. Modelling the transient liquid phase bonding behavior of a duplex stainless steel using copper interlayers [J]. Materials Science Engineering A, 2004, 385: 220-228.
[28] NATSUME Y, OHSASA NARITA T. Phase-field simulation of transient liquid phase ponding process of Ni using Ni-P binary filler metal [J]. Material Transaction, 2003, 44: 819-823.
[29] GHONEIM A, OJO O A. Numerical modeling and simulation of a diffusion-controlled liquid–solid phase change in polycrystalline solids [J]. Computational Material Science, 2011, 50: 1102-1113.
[30] ABBASI K B, ASGHARI G, BAKHTIARI R. TLP bonding of dissimilar FSX-414/IN738 system with MBF80 interlayer: Prediction of solid/liquid interface location [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 996-1003.
Vahid MALEKI1, Hamid OMIDVAR1, Mohammad-reza RAHIMIPOUR2
1. Department of Mining and Metallurgical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Tehran 15875-4413, Iran;
2. Department of Ceramic, Materials and Energy Research Center, Karaj 31787-316, Iran
摘 要:以AMS 4777粉末为填料,研究瞬时液相连接IN-738LC高温合金的显微组织演变,获得完全等温凝固时间。研究间隙大小和连接时间对接头组织的影响。当间隙大小为40 μm时,完全等温凝固时间为45 min。在不完全等温凝固的情况下,连接区的残余液相在非平衡条件下冷却,形成γ-γ共晶相。间隙大小与时间存在非线性关系。随着间隙增大,共晶相变宽。在扩散影响区,发现大量合金元素,其浓度达到峰值,这是由于形成了硼化物和硅化物等金属间化合物。随着间隙增加,所需连接时间增加,合金元素有更多的时间进行扩散和分布至更大区域。因此,合金元素浓度随间隙增加缓慢降低。已有双相模型不能准确预测IN-738LC-AMS 4777-IN738LC瞬时液相连接系统的完全等温凝固时间。
关键词:IN-738LC 高温合金;瞬时液相连接;间隙大小;完全等温凝固
(Edited by Yun-bin HE)
Corresponding author: Hamid OMIDVAR; Tel: +98-21-64542977; E-mail: omidvar@aut.ac.ir
DOI: 10.1016/S1003-6326(16)64132-8

