采用氯铁(II)络离子回收稀水溶液中Au的动力学分析
来源期刊:中国有色金属学报(英文版)2015年第6期
论文作者:Marek WOJNICKI Krzysztof FITZNER Magdalena LUTY-B?OCHO
文章页码:2027 - 2036
Key words:gold recovery; reduction; recycling; electronic waste
摘 要:采用停流法研究氯铁(II)络离子还原氯金(III)络离子的动力学。研究氯金(III)络合离子、Fe2+离子、Cl-离子和中性盐(NaClO4)的初始浓度、溶液pH值和温度的影响。确定激活能为42.36 kJ/mol。此外,提出了动力学方程。采用SEM对所得固相进行分析。粒子直径为0.5~5 μm。结果表明通过还原溶液中氯金(III)络离子并过滤沉淀可以得到Au。
Abstract: The kinetics of Au3+ chloride complex ions reduction using Fe2+ chloride ions was investigated by stopped-flow method. The influence of initial concentrations of Au3+ chloride ions, Fe2+ ions, chloride ions, neutral salt (NaClO4), pH of the solution and temperature was investigated. Activation energy was determined to be 42.36 kJ/mol. Moreover, the kinetic equation was postulated. Obtained solid phase was analyzed using SEM techniques. Particles size varies in the range from 0.5 to 5 μm. It was shown that gold can be removed from the solution by the reduction of Au3+ chloride ions and filtration of precipitant.
Trans. Nonferrous Met. Soc. China 25(2015) 2027-2036
Marek WOJNICKI, Krzysztof FITZNER, Magdalena LUTY-
Faculty of Non-Ferrous Metals, AGH University of Science and Technology, Krakow 30-059, Poland
Received 7 July 2014; accepted 10 November 2014
Abstract: The kinetics of Au3+ chloride complex ions reduction using Fe2+ chloride ions was investigated by stopped-flow method. The influence of initial concentrations of Au3+ chloride ions, Fe2+ ions, chloride ions, neutral salt (NaClO4), pH of the solution and temperature was investigated. Activation energy was determined to be 42.36 kJ/mol. Moreover, the kinetic equation was postulated. Obtained solid phase was analyzed using SEM techniques. Particles size varies in the range from 0.5 to 5 μm. It was shown that gold can be removed from the solution by the reduction of Au3+ chloride ions and filtration of precipitant.
Key words: gold recovery; reduction; recycling; electronic waste
1 Introduction
Since the dawn of the human history, precious metals such as silver and gold have many applications, especially in painting [1], sculpture [2], medicine [3-7] and as a currency (e.g., Polish ducat). However, these metals deposits all over the world are shrinking. It can be predicted that the natural sources of precious metals such as silver, gold, and also platinum and palladium will be exhausted by the end of the year 2030 [8]. This prediction results in an intensive search for new sources of these metals. A well-known alternative for natural sources of precious metals is various wastes, e.g., scrap jewelry [9], used catalysts, mobile phone scraps [10] and electronic waste [11]. The latter is also called as waste of electrical and electronic equipment (WEEE) or e-scrap. Of course, the recovery of precious metals from waste should be cost-effective, easy to use and efficient. Here, we focused on gold, which can generally be recovered by pyrometallurgical, bio- and hydro-metallurgical processes and leaching as demonstrated by SYED [12] and extracted from leachants via adsorption [13,14], cementation [14], ion exchange [14], solvent extraction [14] and reduction. Unfortunately, very often those methods are not selective. During adsorption, other metals such as Cu and Pb can also be removed from the solution [15]. The recovery of gold from secondary sources (waste) is realized by pyro-, hydro-, bio-technologies [12,16]. Generally, all the mentioned technologies are followed by such processes as washing, crushing and separation of metal and plastic. In order to improve the purity of recovered metals, they are converted by repeated dissolution, filtration, and reduction. The reduction process of Au3+ chloride complex ions can be carried out with various reductants such as hydrogen peroxide [17], dimethylamine borane [18], vitamin C [19], sodium citrate [20], as well as using light [21-23]. Depending on the nature of reductants, their properties (weak, strong) and reacting conditions (pH of solution, ionic strength, addition of halide ions, i.e., Cl-), it is possible to obtain metal in different forms like nanoparticles, black deposit, gold sands or flakes. Gold in the form of flakes is especially preferable due to its simple separation from the solution by filtration. Gold recovery from e-scrap seems to be problematic, mostly because of complex composition of this waste. Main board of personal computers (PCs) contains about 86-250 g Au, 694-1000 g Ag, and 110-309 g Pd per ton of waste, and about 18.5%-20% of copper, 2.1%-7% of Fe, 1.3%-5% Al, 4.9%-2.9% Sn, 2.7%-1.5% Pb, 0.4%-1% Ag [24]. Those materials are interesting from the economic point of view, and have to be eliminated from the environmental point of view. However, it is difficult to find a selective method for those metals separation. Application of strong reductants may result in the reduction of less precious metals like copper. Taking this into account, FeCl2+ ions were selected as a reductant of Au3+ complex ions, mostly because of their selectivity and application costs. In Table 1, standard reduction potentials of metals and their ions, commonly appearing in WEEE are gathered. From these data, it is possible to predict if the reduction reaction of Au3+ complex ions by Fe2+ or ferrous complexes may take place.
Table 1 Standard reduction potential at 25 °C
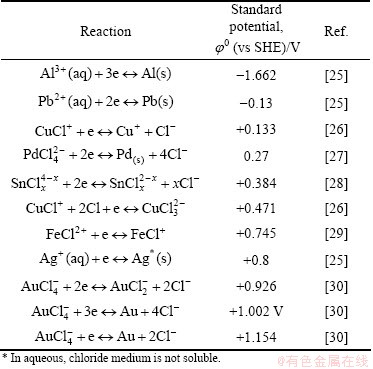
It can be generalized that standard redox potential of complexes is shifted to more negative potential as compared to simple ions [31,32].
Thermodynamic data give only the information about possibility of the reaction occurrence. Thus, any information about reaction kinetic can be obtained only in the experimental way.
2 Experimental
2.1 Reagents
In the experiments, HAuCl4–tetrachloroauric acid was used as a precursor, which was obtained by dissolution of metallic gold (99.99%) in aqua regia (V(HCl):V(HNO3)=1:3). Then, the obtained solution was evaporated several times to remove an excess of nitric acid. Hydrochloric acid (puriss. p.a., 37%, POCH) and perchloric acid (puriss. p.a., 70%, POCH) were used to maintain proper pH and Cl- ions concentration. As a reducing agent, iron dichloride was used (puriss. p.a., >99%). Sodium perchlorate (puriss. p.a.) and sodium chloride (puriss. p.a.) were used to keep the required ionic strength and chloride ions concentrations, respectively. All reagents were dissolved in deionized water (Hydrolab HLP-30 impurities: w(Na+),  w(Cl-), w(Br-),
w(Cl-), w(Br-), 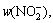
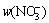 and
and  <0.5× 10-9, w(Fe), w(Zn), w(Cu), w(Cr) and w(Mn)<0.1×10-9, electrical conductivity <0.07 S/cm).
<0.5× 10-9, w(Fe), w(Zn), w(Cu), w(Cr) and w(Mn)<0.1×10-9, electrical conductivity <0.07 S/cm).
2.2 Analytical techniques
Kinetic studies of Au3+ chloride ions reduction were carried out spectrophotometrically using the stopped- flow method (Applied Photophysics, model SX-20 working in the temperature range from 258 to 318 K). Detail information about SX-20 photometer can be found in supporting materials. Solid phase precipitation and coagulation were investigated using UV-Vis spectrophotometer (Shimadzu model U-2501 PC, Japan). Microscopic analyses were carried out using scanning transmission electron microscope (STEM) (Hitachi SU-70, Thermo Science). In this case, the obtained metallic gold particles aqua suspension was droplet on copper grid coated with amorphous carbon layer (thickness of carbon layer about 15 nm), and then the dispersant was evaporated.
3 Results
Using literature data, concentration of Au3+ species appearing in aqueous system was calculated as a function of pH [33] and shown in Fig. 1.
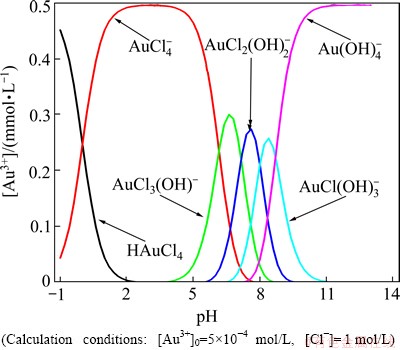
Fig. 1 Forms of Au3+ species in aqueous solution at various pH
It can be seen that at studied pH of 1, only one form of Au3+ complex ion dominates.
UV-Vis spectrum of Fe2+ aqueous solution is shown in Fig. 2. In UV-Vis range, one peak at the wavelength 337 nm can be seen. This peak is overlapping with the peak of gold (maximum absorbance at the wavelength of 314 nm). However, thanks to low molar absorbance of Fe2+ solution, and under isolation condition ([Fe2+]0>> [Au3+]0), we were able to measure the rate of the reduction reaction of Au3+ chloride complex ions using UV-Vis spectrophotometry.
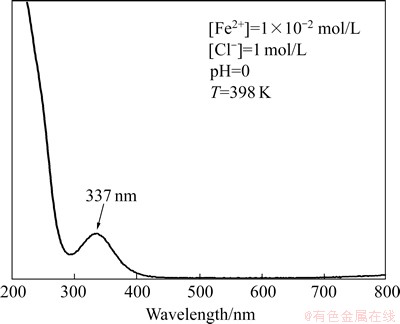
Fig. 2 UV-Vis spectrum of Fe2+ ions in aqueous solution
It is well known that iron ions may hydrolyze with precipitation of insoluble iron hydroxide. In Fig. 3, calculated distribution of iron species is shown [34,35]. It can be seen that in a wide range of pH, Fe2+ ions are stable. However, Fe3+ ions, obtained after the reduction process of Au3+ chloride ions, hydrolyze easily. To prevent this adverse effect, all experiments were carried out at pH equal to either 0 or 1.

Fig. 3 Calculated distribution of Fe2+ and Fe3+ at various pH of solution
In Fig. 4, an example of kinetic curve is shown. It can be seen that the obtained curves approach the limit different from zero. We assumed that this effect is related to the superposition of absorption of UV-Vis light by Au3+ and Fe2+ ions as well as precipitation of gold solid phase.
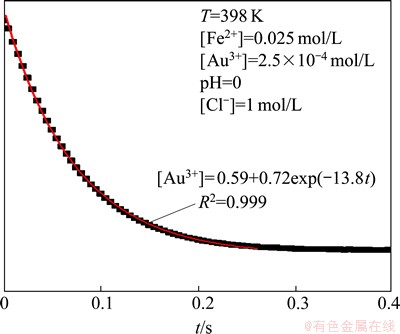
Fig. 4 Example of kinetic curve of Au3+ reduction during reduction reaction
The red line seen in Fig. 4 shows the effect of fitting of the single exponential function to experimental data.
 (1)
(1)
where [Au3+] is the concentration of Au3+; [Au3+]0 is the initial concentration of Au3+; kobs=k·[Fe2+] is the observed reaction rate constant; k is the reaction rate constant; t is time; A is a constant related to the absorbance of Fe2+.
It is worth noting that R2 of the fitted function equals 0.999, which suggests that this equation describes the process well. Equation (1) has been used to determine the values of reaction rate constant.
3.1 Influence of temperature
The influence of temperature has been investigated. For the interpretation of obtained results, two different equations have been applied. The first one is Arrhenius (see supporting materials) dependence and the second one is Eyring-Polanyi equation. Both equations describe the influence of temperature on the reactions rate; however, the first one has an empirical form and the second one is based on the transition state theory.
The Eyring-Polanyi equation can be written in the form:
 (2)
(2)
where ΔG* is the Gibbs activation energy; kB is Boltzmann constant; h is Plank constant; T is the temperature.
Using a logarithmic form of Eq. (2), it is possible to determine the values of ΔS* and ΔH* from the
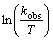 vs 1/T plot (Fig. 5):
vs 1/T plot (Fig. 5):
 (3)
(3)
where ΔS* is the entropy of activation; ΔH* is the enthalpy of activation.

Fig. 5 Graphical determination of activation energy using Eyring-Polanyi equation
From the intercept and the slope of fitted linear equation, the activation energy and the entropy of activation were calculated. The obtained results are gathered in Table 2.
One may see that pH of the solution has significant influence on the activation energy. It may also suggest that pH has an influence on the reaction mechanism. Also, it is important to emphasize that entropy of activation has a negative sign for the studied reaction. On one hand, it may suggest that the structure of the obtained product is more ordered than the substrate. On the other hand, negative value of entropy suggests that the number of degrees of freedom related to rotation and translation of species decreases. STAVILA et al [36] suggest that this effect may be due to the reactants binding to the surface. It can be seen that pH also has influence on the value of the entropy of activation.
3.2 Influence of reductant initial concentration
The influence of initial concentration of reducing agent on reaction kinetics was investigated. The experiments were carried out at pH values of 0 and 1, with constant concentration of chloride and Au3+ chloride ions, and at constant value of temperature.
The obtained dependences shown in Fig. 6 are linear and intercept at (0, 0) point. It confirms that the accepted assumption (kobs=k·c0(Fe2+)) is correct in the range of initial concentrations applied in this work. The values of the slopes for pH=0 and pH=1 are equal to (975±6) and (626 ±44), respectively.
3.3 Order of reaction
To determine the reaction order in respect to Au3+ ions initial concentration, the initial rate method was applied. It is well known that the reaction rate can be expressed as
 (4)
(4)
And under isolation condition, Eq. (4) can be rearranged into the form:
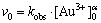 (5)
(5)
Using a logarithmic form of Eq. (5), it is possible to determine the order “α” of the reaction from the slope of fitted linear dependences. It results from the plots shown in Fig. 7 that the order of the reaction can be assumed as 1.
The influence of pH on the order of the reaction can be noted (see Fig. 7). For the pH equal to 1, the order of the reaction in respect to Au3+ chloride complex ions can be assumed as 1. However, at pH equal to 0, it is lower and equal to 0.80±0.02. This difference in order of the reaction suggests that the studied process is complex and consists of several parallel reactions. This phenomenon can be explained as an effect of Au3+ chloride complex dissociation reaction, which can be described as
HAuCl4
 (6)
(6)
where k0 denotes equilibrium constant and it equals 1 [37].
Table 2 Influence of experimental conditions on value of parameters in Eyring-Polanyi equation


Fig. 6 Influence of initial concentration of reductant on observed reaction rate constant
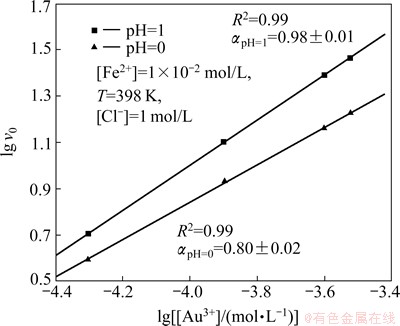
Fig. 7 Determination order of reaction using initial rate method
As it can be seen that the increase of H+ concentration causes a decrease of 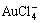 concentration (shift of reaction equilibrium to the left side of Eq. (6).
concentration (shift of reaction equilibrium to the left side of Eq. (6).
Then, the initial rate of the reaction can be expressed as
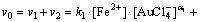
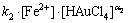 (7)
(7)
Assuming that the order of the reaction in respect to not dissociated Au3+ chloride complex is also equal to 1, taking into account Eq. (6) and isolation conditions, Eq. (7) can be transformed as
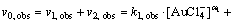
 (8)
(8)
Finally, Eq. (8) can be expressed as
 (9)
(9)
As can be seen (Eq. (6)) at pH=1, dissociated form of Au3+ dominates, while for pH=0, c.a. half Au3+ complex dissociated and half did not dissociate. Bearing in mind dependences kobs vs c0(Fe2+) and Eq. (9), we may come to the conclusion that determined values of the slope for pH=0 and pH=1 are proportional to the following equations system:
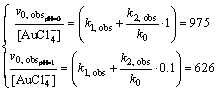 (10)
(10)
It follows from Eq. (10) that k1,obs=588 mol-1·L·s-1 and k2,obs=388/(mol-1·L·s-1). It can be seen that the rate constant for Au3+ chloride complex ions reduction is greater than that for molecular Au3+ chloride complex.
3.4 Influence of neutral salt concentration on reaction kinetics
It is well known that ionic strength of the solution has an influence on the reaction kinetics. This effect described for the first time by  in 1922 and later modified by Bjerrum, is now called
in 1922 and later modified by Bjerrum, is now called  -Bjerrum primary salt effect. It can be expressed as
-Bjerrum primary salt effect. It can be expressed as
 (11)
(11)
where  is the constant value and for aqueous solution, it is equal to 0.509; ZA and ZB are the charges of reactants; I is ionic strength; k0 is rate constant at ionic strength equal to 0.
is the constant value and for aqueous solution, it is equal to 0.509; ZA and ZB are the charges of reactants; I is ionic strength; k0 is rate constant at ionic strength equal to 0.
As it was shown above, studied system is complex and it consists of at least two parallel reactions. In a case where molecular complex of Au3+ chloride is reacting with Fe2+ ions, no salt effect is expected. For the reaction of Au3+ chloride complex ions with Fe2+ such effect is expected (see Fig. 8).

Fig. 8 Salt effect on reaction kinetics
By matching Eq. (11) to the experimental data, the value of the slope was obtained as -0.12 (see Fig. 8). The negative sign of the slope suggests that in the studied system, Au3+ chloride complex reacts with positively charged ions (in this case Fe2+); however, the small value of the slope may be due to the superposition of reduction reactions by Fe2+ and other negatively charged species. Complexes of iron which can crystallize readily as Na2FeCl4·2H2O [38] are well known, e.g.,  Fe2+ chloride complex can be obtained from hydrochloric acid solutions containing alkali metal chlorides. In the case of our experiments, ionic strength was set up by the addition of NaClO4 while Cl- concentration was adjusted by using NaCl. It leads us to the conclusion that Au3+ chloride complex was reduced by Fe2+ ions and also by Fe2+ chloride complex [39].
Fe2+ chloride complex can be obtained from hydrochloric acid solutions containing alkali metal chlorides. In the case of our experiments, ionic strength was set up by the addition of NaClO4 while Cl- concentration was adjusted by using NaCl. It leads us to the conclusion that Au3+ chloride complex was reduced by Fe2+ ions and also by Fe2+ chloride complex [39].
3.5 Influence of Cl- concentration
The influence of Cl- on the reaction kinetics was studied. The Cl- concentration was adjusted using sodium chloride. To maintain pH=0, perchloric acid was used. The results of the experiment are shown in Fig. 9.
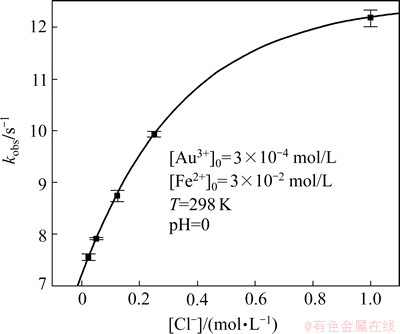
Fig. 9 Influence of Cl- concentration on observed reaction rate constant
It can be seen that an increase of chloride ions concentration leads to the increase of kobs. In the studied range of chloride ions concentration, the dependency of observed rate constant vs Cl- concentration can be described by the equation in the following form:
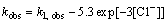 (12)
(12)
where k1, obs is the observed rate constant in the case of reduction of Au3+ chloride complex ions by Fe2+ complex.
In the limit, this function for [Cl-] approaching infinity yields k1,obs. The function must be restricted by the solubility limit, i.e., about 6 mol/L for NaCl at 298 K. It can be suggested that the increase of chloride ions concentration leads to the formation of Fe2+ chloride complex, which results in an increase of reaction rate.
In such case, kobs, for initial concentration of [Cl-]=0, corresponds to reduction rate constant for [Fe2+] ions and is equal to 5.17.
It should be pointed out that the influence of chloride ions concentration strongly depends on the applied reductant. For example, in the case of S4+ [40,41], a negligible effect was observed in the range of Cl- concentration from 0.05 to 1.55 mol/L. The negative effect of Cl- ions was observed in case of Au3+ reduction with dimethylamine borane and vitamin C [18,19]. Such observations suggest that the increase of chloride ions concentration results in a new complex formation.
3.6 Solid phase formation
The process of solid phase formation and the morphology of the obtained deposit were also investigated. Photos of the obtained samples are shown in Fig. 10.
Gold flakes appear on the surface of the solution a few minutes after the reaction takes place. A similar phenomenon was noticed by PARINAYOK et al [42]; however, they carried out their experiment at pH=6.
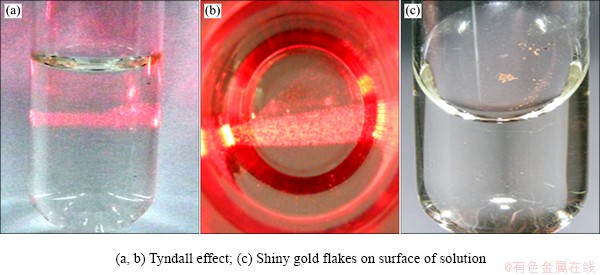
Fig. 10 View of sample after reduction process
Morphology of the obtained gold particles was investigated using SEM microscopy. In Fig. 11, the SEM image of gold particles is shown.

Fig. 11 SEM image of obtained solid phase of gold
The shape of some conglomerates is similar to a cauliflower. The average size of the particles depends on the experimental conditions. Thanks to this, the obtained gold flakes can be easily separated from the solution by filtration.
In Fig. 12, an example of kinetic curves of solid phase precipitation is shown.
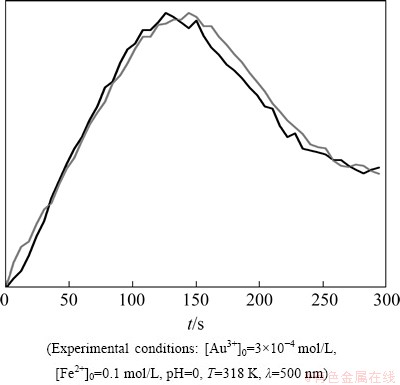
Fig. 12 Kinetic curves presenting process of solid phase precipitation
Because of low absorbance level, obtained results were normalized to 1. Under experimental conditions, the maximum absorbance (measured at the wavelength of 500 nm) can be followed by 150 s. After this time, the absorbance quickly disappears.
Also, the influence of temperature on the particles size and shape was investigated. It can be seen (Fig. 13) that the shape strongly depends on temperature. At the temperature of 288 K, particles have a shape close to pyramid with some deformation. Increase of temperature results in the formation of irregular particles.
3.7 Solid phase analysis
The picture of gold particles obtained during Au3+ ions reduction using Fe2+ ions and the results of analysis are shown in Fig. 14. One may see in Fig. 14(b) that no peak was observed due to Fe. It confirms that during the reduction process of Au3+, Fe2+ or Fe3+ ions are not bonded to metallic gold. Some peaks may be seen due to copper and aluminum. Those peaks are artifacts, coming from the support and grid used during the analysis.

Fig. 13 Influence of temperature on particle size and shape

Fig. 14 View of gold particles precipitated during Au3+ ions reduction with Fe2+ (a) and analysis of precipitate (b)
3.8 Summary
Taking into account the obtained results, the reduction reaction scheme can be postulated according to the following steps:
 (13)
(13)
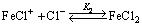 (14)
(14)
where K1 and K2 denote equilibrium constants for the reactions (13) and (14), respectively.
 (15)
(15)
 (16)
(16)
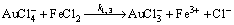 (17)
(17)
 (18)
(18)
 (19)
(19)

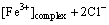 (20)
(20)
We can assume that the rate constants k1, 1, k1, 2, k1, 3>>k4 (Fig. 4 and 12 and Eqs. (15)-(17)), since the Au3+ chloride complex is extremely unstable [43]. Moreover, we can assume that the rate constant of Au2+ disproportionation k2 is greater than k3. GAMMONS et al [44] reported that disproportionation rate constant of Au+ can be expressed by the following polynomial function of temperature:
 (21)
(21)
where T is expressed in Kelvin. Also, they point out that for the first 5-10 min, the rate of this process is very slow. Moreover, the disproportionation process is catalyzed by metallic gold. In the case of our experiments, in less than 2 s, reduction reaction takes place (Fig. 4), and after less than 1 min, solid phase was observed (Fig. 12). This suggests that Au+ is further reduced to metallic form by Fe2+. By using spectrophotometric method, the changes of Au3+ ions concentration were investigated, the differential equation describing the process of Au3+ consumption can be written as

 (22)
(22)
where x, y and z denote unknown order of the reaction in respect to Fe2+ and Fe2+ complexes. Equation (22) can be expressed as
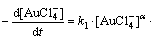
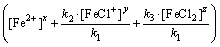 (23)
(23)
As it was mentioned before, under isolation conditions, the changes of Fe2+ ions and complexes concentration can be neglected. Therefore, Eq. (23) can be given in the following form:
 (24)
(24)
where
 (25)
(25)
Moreover, it was shown that at low pH, molecular form of Au3+ chloride complex appears.
It should be pointed out that the order of the reaction in respect to Fe2+ total initial concentration is equal to 1 (see Fig. 6). It is demonstrated that α=1 (see Fig. 7). Consequently, the integral form of Eq. (24) is
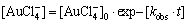 (26)
(26)
which is identical to the equation assumed above to determine reaction rate constant.
The impact of Cl- concentration was studied. In the kinetic equation, this effect is related to Fe2+ complexes and related to their equilibrium constants.
It seems that gold recovery from electronic waste is possible using Fe2+ chloride as a reducing agent. Importantly, this process seems to be selective from the thermodynamic point of view. Reduction of Ag, Au, Cu and Hg to the metallic form has been described by Fe2+/Fe3+ hydroxysulfate green rust [45]; however, it seems that this process can be negligible.
It is difficult to explain the differences in the obtained data and data presented by MODLEY and NICOLS [46].They have reported that at a short time (1-2 h), no evidence of the formation of metallic gold was observed.
4 Conclusions
1) Significant influence of the chloride ions concentration on the reaction rate was observed. The increase of the reaction rate was explained as the impact of chloride ions on the formation of Fe2+ chloride complex.
2) Activation energy of the process was determined and equals 47.69 kJ/mol at the experimental conditions: [Au3+]0=3×10-4 mol/L, [Fe2+]0=3×10-2 mol/L, [Cl-]=1 mol/L, pH=0 and 37.21 kJ/mol at pH=1. Differences in the activation energy between pH 0 and 1 suggest that the studied process is complex. At least two different paths of the reduction reaction of Au3+ are possible. It is strongly suggested that the reducing agent Fe2+ consists of two forms, Fe2+ ions and Fe2+ chloride complex ions. In such case, the differences in activation energy at different pH can be explained as the changes in concentration of Fe2+ complex ions and Fe2+ ones. Those changes in concentration of reductants result in changes of contribution of each elementary reaction in the total reaction rate.
3) Reduction of Au3+ chloride complex by Fe2+ chloride ions leads to the formation of metallic gold.
Acknowledgments
This work was supported by the European Grant No. POIG.01.01.02.-00-015/09-00. The authors are grateful to DR Tomasz TOKARSKI from the Department of Structure and Mechanics of Solids, Faculty of Non-ferrous Metals of AGH University of Science and Technology for his help during SEM analysis.
References
[1] FREESTONE I, MEEKS N, SAX M, HIGGITT C. The Lycurgus cup-A roman nanotechnology [J]. Gold Bulletin, 2007, 40(4): 270-277.
[2] VERMEULE C C. Museum of fine arts B. Greek and Roman sculpture in gold and silver [M]. Boston: Museum of Fine Arts, 1974.
[3] HIGBY G. Gold in medicine [J]. Gold Bulletin, 1982, 15(4): 130-140.
[4] GUO Z, SADLER P J. Metals in medicine [J]. Angewandte Chemie International Edition, 1999, 38(11): 1512-1531.
[5] RIEHEMANN K, SCHNEIDER S W, LUGER T A, GODIN B, FERRARI M, FUCHS H. Nanomedicine—Challenge and perspectives. [J] Angewandte Chemie International Edition 2009, 48(5): 872-897.
[6] DREADEN E C, MACKEY M A, HUANG X, KANGY B, EL-SAYED M A. Beating cancer in multiple ways using nanogold [J]. Chem Soc Rev, 2011, 40: 3391-3404.
[7] CHERNOUSOVA S, EPPLE M. Silver as antibacterial agent: Ion, nanoparticle, and metal [J]. Angewandte Chemie International Edition, 2013, 52(6): 1636-1653.
[8] MOYER M. How much is left? [J]. Scientific American, 2010, 303(3): 74-81.
[9] POTGIETER J H, POTGIETER S S, MBAYA R K K, TEODOROVIC A. Small-scale recovery of noble metals from jewellery wastes [J]. Journal of The South African Institute of Mining and Metallurgy, 2004, 104(10): 563-571.
[10] LI Jing-ying, XU Xiu-li, LIU Wen-quan. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones [J]. Waste Management, 2012, 32(6): 1209-1212.
[11] CHMIELEWSKI A G,  W. Separation technologies for metals recovery from industrial wastes [J]. Hydrometallurgy, 1997, 45(3): 333-344.
W. Separation technologies for metals recovery from industrial wastes [J]. Hydrometallurgy, 1997, 45(3): 333-344.
[12] SYED S. Recovery of gold from secondary sources-A review [J]. Hydrometallurgy, 2012, 115-116: 30-51.
[13]  K, WOJNICKI M. Kinetics of the adsorption of Au3+ chloride complex ions onto activated carbon [J]. Archives of Metallurgy and Materials, 2009, 54(3): 853-560.
K, WOJNICKI M. Kinetics of the adsorption of Au3+ chloride complex ions onto activated carbon [J]. Archives of Metallurgy and Materials, 2009, 54(3): 853-560.
[14] CUI J, ZHANG L. Metallurgical recovery of metals from electronic waste: A review [J]. Journal of Hazardous Materials, 2008, 158(2-3): 228-256.
[15] FAUR-BRASQUET C, KADIRVELU K, CLOIREC P L. Removal of metal ions from aqueous solution by adsorption onto activated carbon cloths: adsorption competition with organic matter [J]. Carbon, 2002, 40: 2387-2392.
[16] GRAMATYKA P, NOWOSIELSKI R, SAKIEWICZ P. Recycling of waste electrical and electronic equipment [J]. Journal of Achievements in Materials and Manufacturing Engineering, 2007, 20(1-2): 535-538.
[17]  K, FITZNER K. Kinetics of reduction of Au3+ complexes using H2O2 [J]. Metallurgical and Materials Transactions B, 2006, 37(5): 703-714.
K, FITZNER K. Kinetics of reduction of Au3+ complexes using H2O2 [J]. Metallurgical and Materials Transactions B, 2006, 37(5): 703-714.
[18] WOJNICKI M, RUDNIK E,  K, FITZNER K. Kinetic studies of Au3+ chloride complex reduction and solid phase precipitation in acidic aqueous system using dimethylamine borane as reducing agent [J]. Hydrometallurgy, 2012, 127-128: 45-53.
K, FITZNER K. Kinetic studies of Au3+ chloride complex reduction and solid phase precipitation in acidic aqueous system using dimethylamine borane as reducing agent [J]. Hydrometallurgy, 2012, 127-128: 45-53.
[19]  K, WOJNICKI M, FITZNER K. The kinetics of redox reaction of Au3+ chloride complex ions with L-ascorbic acid [J]. Inorganica Chimica Acta, 2013, 395: 189-196.
K, WOJNICKI M, FITZNER K. The kinetics of redox reaction of Au3+ chloride complex ions with L-ascorbic acid [J]. Inorganica Chimica Acta, 2013, 395: 189-196.
[20] TURKEVICH J, STEVENSON P C, HILLIER J. The formation of colloidal gold [J]. J Phys Chem, 1951, 57(7): 670-673.
[21] KWOLEK P, WOJNICKI M. The kinetic study of photoreduction of tetrachloroaurate acid by methanol in acidic media [J]. Journal of Photochemistry and Photobiology A, 2014, 286: 47-54.
[22] WOJNICKI M, TOKARSKI T, KWOLEK P. Kinetic study of the photoelectrochemical gold recovery from diluted chloride solutions [J]. Archives of Metallurgy and Materials, 2013, 58(3): 709-716.
[23] MOHAMED H H, DILLERT R, BAHNEMANN D W. Kinetic and mechanistic investigations of the light induced formation of gold nanoparticles on the surface of TiO2 [J]. Chem Eur J, 2012, 18: 4314-4321.
[24] TUNCUK A, STAZI V, AKCIL A, YAZICI E Y, DEVECI H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling [J]. Minerals Engineering, 2012, 25(1): 28-37.
[25] ZOSKI C G. Handbook of electrochemistry [M]. Amsterdam: Elsevier Science & Technology, 2007.
[26]  O. Redox potential characteristics of cupric chloride solutions [J]. Hydrometallurgy, 2009, 95(3-4): 285-289.
O. Redox potential characteristics of cupric chloride solutions [J]. Hydrometallurgy, 2009, 95(3-4): 285-289.
[27] MECH K,  P, KOWALIK R, FITZNER K. Voltammetric study of electro-reduction of tetraamminepalladium(II) onto gold electrode [J]. Journal of Electroanalytical Chemistry, 2012, 685(1): 15-20.
P, KOWALIK R, FITZNER K. Voltammetric study of electro-reduction of tetraamminepalladium(II) onto gold electrode [J]. Journal of Electroanalytical Chemistry, 2012, 685(1): 15-20.
[28] OECD. Chemical thermodynamics of tin [M]. Volume 12. Paris: OECD Publishing, 2012.
[29] WHITE R, TRAINHAM J A, NEWMAN J, CHAPMAN T W. Potential-selective deposition of copper from chloride solutions containing iron [J]. Journal of The Electrochemical Society, 1977, 124(5): 669-676.
[30] LINGANE J J. Standard potentials of half-reactions involving + 1 and + 3 gold in chloride medium: Equilibrium constant of the reaction AuCl4-+2Au+2Cl-=3AuCl2- [J]. Journal of Electroanalytical Chemistry, 1962, 4(6): 332-342.
[31] MECH K,  P, KOWALIK R. Analysis of rhodium electrodeposition from chloride solutions [J]. Journal of The Electrochemical Society, 2014, 161(9): D458-D461.
P, KOWALIK R. Analysis of rhodium electrodeposition from chloride solutions [J]. Journal of The Electrochemical Society, 2014, 161(9): D458-D461.
[32] MECH K,  P, KOWALIK R. Co-reduction of electrochemically active [Co(H2O)6]2+ and [CoCl(H2O)5]+ complexes onto gold electrode [J]. Journal of The Electrochemical Society 2013, 160(6): D246-D250.
P, KOWALIK R. Co-reduction of electrochemically active [Co(H2O)6]2+ and [CoCl(H2O)5]+ complexes onto gold electrode [J]. Journal of The Electrochemical Society 2013, 160(6): D246-D250.
[33] GMELIN L. Gmelin handbook of inorganic and organometallic chemistry [M]. Berlin: Springer-Verlag, 1992.
[34] MULAY L N, SELWOOD P W. Hydrolysis of Fe3+: Magnetic and spectrophotometric studies on ferric perchlorate solutions [J]. J Am Chem Soc, 1955, 77(10): 2693-2701.
[35] GAYER K H, WOOTNER L. The hydrolysis of ferrous chloride at 25 °C [J]. J Am Chem Soc, 1956, 78(16): 3944-3946.
[36] STAVILA V, VOLPONI J, KATZENMEYER A M, DIXON M C, ALLENDORF M D. Kinetics and mechanism of metal–organic framework thin film growth: Systematic investigation of HKUST-1 deposition on QCM electrodes [J]. Chemical Science 2012, 3: 1531-1540.
[37] SEN GUPTA K K, PAL B, BEGUM B A. Reactivity of some sugars and sugar phosphates towards Au3+ in sodium acetate–acetic acid buffer medium [J]. Carbohydrate Research, 2001, 330(1): 115-123.
[38] WIBERG E, WIBERG N, HOLLEMAN A F. Inorganic chemistry [M]. San Diego: Academic Press, 2001.
[39]  A. The basics of inorganic chemistry [M]. Warszawa: PWN SA, 2010.
A. The basics of inorganic chemistry [M]. Warszawa: PWN SA, 2010.
[40]  K, FITZNER K. Kinetics of Au3+ chloride complex reduction using S4+ [J]. Metallurgical and Materials Transactions B, 2004, 35(6): 1071-1085.
K, FITZNER K. Kinetics of Au3+ chloride complex reduction using S4+ [J]. Metallurgical and Materials Transactions B, 2004, 35(6): 1071-1085.
[41] GUPTA K K S, DAS S, GUPTA S S. Kinetic of the oxidation of sulphite by tetrachloroaurate(III) [J]. Transition Met Chem, 1988, 13: 261-263.
[42] PARINAYOK P, YAMASHITA M, YONEZU K, OHASHI H, WATANABE K, OKAUE Y, YOKOYAMA T. Interaction of Au3+ and Pt4+ complex ions with Fe2+ ions as a scavenging and a reducing agent: A basic study on the recovery of Au and Pt by a chemical method [J]. Journal of Colloid and Interface Science 2011, 364(1): 272-275.
[43] BARAKAT K A, CUNDARI T R,  H, OMARY M A. Disproportionation of Au2+ complexes: A density functional study of ligand and solvent effects [J]. Journal of Physical Chemistry B, 2006, 110(30): 14645-14651.
H, OMARY M A. Disproportionation of Au2+ complexes: A density functional study of ligand and solvent effects [J]. Journal of Physical Chemistry B, 2006, 110(30): 14645-14651.
[44] GAMMONS C H, YU Y, WILLIAMS-JONES A E. The disproportionation of Au(I) chloride complexes at 25 to 200 °C [J]. Geochimica et Cosmochimica Acta, 1997, 61(10): 1971-1983.
[45] O’LOUGHLIN E J, KELLY S D, KEMNER K M, CSENCSITS R, COOK R E. Reduction of AgI, Au3+, Cu2+, and HgII by Fe2+/Fe3+ hydroxysulfate green rust [J]. Chemosphere, 2003, 53(5): 437-446.
[46] MOODLEY K G, NICOLS M J. Kinetics of reduction of Au3+ by platinum(II) and Fe3+ in aqueous chloride solutions [J]. Journal of the Chemical Society, Dalton Transactions, 1977(10): 993-996.
Marek WOJNICKI, Krzysztof FITZNER, Magdalena LUTY-
Faculty of Non-Ferrous Metals, AGH University of Science and Technology, Krakow 30-059, Poland
摘 要:采用停流法研究氯铁(II)络离子还原氯金(III)络离子的动力学。研究氯金(III)络合离子、Fe2+离子、Cl-离子和中性盐(NaClO4)的初始浓度、溶液pH值和温度的影响。确定激活能为42.36 kJ/mol。此外,提出了动力学方程。采用SEM对所得固相进行分析。粒子直径为0.5~5 μm。结果表明通过还原溶液中氯金(III)络离子并过滤沉淀可以得到Au。
关键词:金回收;还原;回收;电子废料
(Edited by Yun-bin HE)
Corresponding author: Marek WOJNICKI; Tel/Fax: +48-12-6174126; E-mail: marekw@agh.edu.pl
DOI: 10.1016/S1003-6326(15)63812-2

