文章编号:1004-0609(2016)-06-1324-08
CO还原焙烧铁酸锌的选择性分解行为
韩俊伟1,刘 维1,覃文庆1,蔡 鑫2,罗虹霖1,王大伟1
(1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 冶金与环境学院,长沙 410083)
摘 要:为了将锌焙砂中铁酸锌选择性地分解为ZnO和Fe3O4,研究在CO还原焙烧过程中铁酸锌的分解行为。采用HSC和Factsage软件计算铁酸锌在CO还原气氛下分解的热力学基础,再通过回转窑焙烧试验考察还原焙烧条件对铁酸锌分解行为的影响。结果表明:在适宜的温度和气氛下锌焙砂中的铁酸锌能选择性转化为ZnO和Fe3O4,CO浓度、p(CO)/p(CO+CO2)值、焙烧温度和时间是影响铁酸锌分解的主要因素,提高焙烧温度、延长时间、增加CO浓度和分压有利于铁酸锌的分解,也会促进FeO的生成;在最佳条件下,铁酸锌的分解率近70%,且过还原不严重。经XRD和SEM/EDS分析,产物主要以ZnO、Fe3O4、ZnS和Zn2SiO4为主,且颗粒粒度较小、疏松多孔及互相包裹严重。
关键词:铁酸锌;锌焙砂;锌浸渣;还原焙烧;分解行为
中图分类号:TD952 文献标志码:A
近年来,我国锌金属产量和消费量均居世界第一,2013年产量高达530万t,其中70%以上通过“氧化焙烧-低酸浸出-净化-电积”常规湿法炼锌工艺产出[1-3]。在氧化焙烧过程中,锌精矿伴生的大部分杂质铁不可避免地会与氧化锌反应生成铁酸锌(ZnFe2O4)[4-5]。铁酸锌结构稳定、不溶于弱酸,不仅导致后续锌浸出率低,而且产生大量以铁酸锌为主的浸出渣,据估算每生产1万t电解锌产出1~1.05万t浸出渣,渣中含有大量的重金属元素,如Zn、Fe、Pb、Ga和Ag等,不仅浪费了宝贵的金属资源,而且长期堆放还会严重污染环境[6-7]。因此,有效回收锌浸渣中的有价金属,既有利于缓减资源供需矛盾,又有利于环境保护。
针对锌浸渣中有价金属回收的问题,国内外学者进行了大量研究。20世纪60年代以前,锌浸渣主要采用火法工艺处理[8-9],如回转窑烟化法和电炉炼锌法等。这些火法处理工艺大都存在能耗高、流程长和贵金属回收率低等缺点。20世纪60年代后期,锌浸渣的处理逐步转为热酸浸出的湿法工艺,包括黄钾铁矾法[10-12]、针铁矿法[13-14]和赤铁矿法[15]等。热酸浸出法成功解决锌浸出率低和有价金属铅银回收难的问题,但存在着除铁负担繁重、操作复杂、生产成本高、有价元素随铁渣损失大、铁资源不能有效利用和铁渣中存在大量不稳定重金属污染物造成二次污染等缺点。近年来,一些学者们提出了火法湿法联合处理的新工艺,即通过焙烧的方法将锌浸渣中的铁酸锌转化为易溶锌物相,再通过中性或低酸浸出工艺回收锌,比如转化焙烧法[16-17]、硫酸化焙烧法[18]和还原焙烧法[19-21]等。虽然这些新方法在减少能耗和环境保护等方面存在巨大的潜力,但仍处于试验阶段,许多问题有待解决。
针对锌浸渣量大的问题,大量的研究与应用是从治理的角度着手,较少从预防的角度考虑。本课题组秉着综合回收的思想,提出了在常规湿法炼锌的氧化焙烧与低酸浸出过程之间增加还原焙烧工序[22],由于氧化焙烧的温度在900 ℃以上,还原焙烧在相对低温下进行,这样就可以利用氧化焙砂所带的余热进行还原焙烧,将锌焙砂中的铁酸锌选择性的分解为氧化锌和四氧化三铁,生成的氧化锌在后续的低酸浸出工序中溶解于浸出液,而磁铁矿不溶于弱酸而富集于浸出渣中,再通过多级磁选工艺回收铁,同时伴生的贵金属(如Pb和Ag)富集于磁选尾矿中,方便进一步回收。这样不仅可以综合回收锌焙砂中的有价金属和降低浸出渣产量,还能省去后续腐蚀性强的热酸浸出和繁杂的沉铁工序。由于该工艺成败的关键是锌焙砂中的铁酸锌能否在低温下实现选择性分解,所以本文作者主要研究铁酸锌在CO还原气氛下的选择性分解行为。
1 实验
1.1 试验原料
本试验中所用原料为内蒙古某湿法炼锌厂的锌焙砂,其化学成分组成、锌铁物相组成和XRD分析结果分别如表1、表2和图1所示。由表1可知,该样品主要含Zn和Fe两种有价元素,其次为Pb、Cu、Mn、Cd等,还有一定量的Ag。从XRD谱可知,锌焙砂中主要含有ZnO、ZnFe2O4、Fe3O4、ZnS和Zn2SiO4等物相。由于铁酸锌和四氧化三铁的XRD峰几乎重叠,且受氧化焙烧的影响,锌焙砂中物相的晶体结构可能与天然矿物存在略微的差别,因此,仅根据XRD无法区分二者,这给试验研究增加难度。原样的化学物相分析结果(见表2)表明,锌主要以ZnO和ZnFe2O4的形式存在,含量超过了总量的96%;铁主要以ZnFe2O4的形式存在,占总铁含量的82.53%。因此,该试样为典型的高铁锌焙砂。此外,本试验中所用的还原剂是CO气体,辅助气体有N2和CO2,N2主要用作补充气体和保护气体,CO2主要作用是控制反应气氛的还原强度。
1.2 试验方法
预先将锌焙砂磨细至0.074 mm以下,并烘干供试验使用。每次试验称取120g样品放入间断性密闭回转窑[23]配套的刚玉罐中,再将装有样品的刚玉罐安装于回转窑中进行焙烧。先在N2保护下加热回转窑至刚玉罐内温度达到设定值,再通入合适流量的CO和CO2气体,恒温焙烧一定时间后停止通入CO和CO2,同时停止加热,在N2保护下冷却至室温后取出样品。最后采用XRD和SEM分析焙烧产物的物相组成和微观形貌特征,化验产物中ZnFe2O4及Fe2+和全铁(FeT)的含量,并计算铁酸锌的分解率和w(Fe2+)/w(FeT)的值。
本试验中采用铁酸锌的分解率和产物中w(Fe2+)/w(FeT)的比值评价锌焙砂中铁酸锌选择性分解的效果。根据前期探索实验,在该实验条件下锌焙砂中的铁酸锌基本不会被还原为单质铁,生成的铁物相以氧化物为主,因此w(Fe2+)/w(FeT)值主要反映了焙烧产物中铁物相的还原程度。假设焙烧产物中铁全部以Fe3O4形式存在,则w(Fe2+)/w(FeT)=1/3;如果产物中w(Fe2+)/w(FeT)<1/3,说明铁酸锌分解不彻底;如果产物中1/3<w(Fe2+)/w(FeT)<1,表明部分铁物相过还原为FeO。
表1 锌焙砂化学成分

表2 锌焙砂的锌、铁物相组成

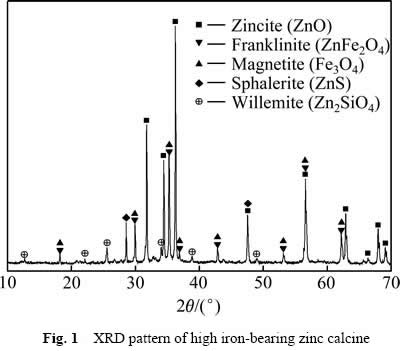
图1 高铁锌焙砂的XRD谱
2 结果与讨论
2.1 铁酸锌还原热力学分析
吉布斯自由能变化(?G)是化学反应能否发生的第一判据,只有当反应的?G<0时,该反应才能发生。在CO还原铁酸锌过程中可能发生的主要反应如式(1)~(5)所示,采用HSC Chemistry 5.0软件计算了各反应的标准吉布斯自由能变化( )随温度的变化关系,结果如图2所示。
)随温度的变化关系,结果如图2所示。
3ZnFe2O4+CO(g)=3ZnO+2Fe3O4+CO2(g) (1)
ZnFe2O4+CO(g)=ZnO+2FeO+CO2(g) (2)
Fe3O4+CO(g)=3FeO+CO2(g) (3)
FeO+CO(g)=Fe+CO2(g) (4)
ZnO+CO(g)=Zn+CO2(g) (5)
由图2可知,反应(1)的标准吉布斯自由能变化在100~1000 ℃温度范围内恒为负,且随着温度的增加,反应的吉布斯自由能变化呈下降趋势,说明CO能将铁酸锌还原为氧化锌和四氧化三铁,且提高温度可以促进反应的发生。反应(2)的标准吉布斯自由能变化随温度的增加逐渐降低,在温度高于250℃后,铁酸锌在标准状态下可以被还原为氧化锌和氧化亚铁。反应(3)的标准吉布斯自由能的变化值随着温度的增加由正逐渐变负,且700 ℃为转变点,表明提高温度能使四氧化三铁还原为氧化亚铁。反应(2)和反应(3)的发生会导致易溶的非磁性的氧化亚铁的产生,不利于铁酸锌选择性的分解为氧化锌和四氧化三铁。由于在100~1000 ℃温度范围内,反应(1)可能发生的趋势明显大于反应(2)和反应(3)的,因此,可以通过调节焙烧温度使铁酸锌选择性的分解为氧化锌和四氧化三铁,避免铁酸锌和四氧化三铁的过还原。此外,在标准状态下,氧化亚铁在600 ℃以上不能被还原为单质铁,氧化锌在1000 ℃以下部不会过还原为单质锌。
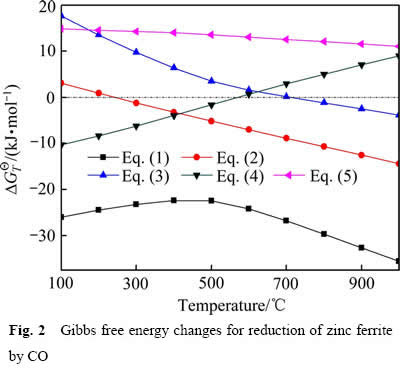
图2 CO还原铁酸锌的标准吉布斯自由能变化
为了进一步从热力学上研究还原气氛和温度对铁酸锌分解产物的影响,采用Factsage软件计算了铁酸锌在650~900 ℃温度范围内的优势区域,其结果如图3所示。假定反应体系总压为101 kPa。由图3可以看出,当CO和CO2分压在10.1 Pa~101 kPa范围内,随着CO分压的增加、CO2分压的降低,铁酸锌还原产物的平衡物相依次为ZnFe2O4+ZnO、Fe3O4+ZnO、FeO+ZnO、Fe+ZnO、和Fe3C+ZnO。随着温度的增加,ZnFe2O4+ZnO和Fe3C+ZnO区域逐渐缩小,FeO+ZnO和Fe+ZnO区域逐渐增大,Fe3O4+ZnO区域未发生明显变化,一直保持较广的优势区域,这有利于铁酸锌选择性地还原为氧化锌和四氧化三铁,说明CO2的添加有助于控制铁物相的过还原。由此可见,CO和CO2的分压比是实现铁酸锌的选择性分解的关键,焙烧温度对铁酸锌的还原过程也有一定的影响,温度过低不利于铁酸锌的分解,温度过高则有助于氧化亚铁的生成。因此,从热力学上讲,当温度在适宜的范围内,通过调节CO和CO2的分压比可以实现铁酸锌的选择性分解。
2.2 焙烧条件对铁酸锌分解行为的影响
为了考察还原焙烧条件对锌焙砂中铁酸锌选择性分解行为的影响,本实验中采用控制变量法系统地研究了各因素对铁酸锌分解率和焙烧产物w(Fe2+)/w(FeT)值的影响,其结果如图4所示。根据前期探索试验,设定初始条件为:CO浓度5%(体积分数)、p(CO)/p(CO+CO2)=0.2、气体流量1 L/min、焙烧温度700 ℃、回转窑转速5 r/min及焙烧时间1 h,在进行单因素试验时除了考察因素变化外其他条件均为该初始值。
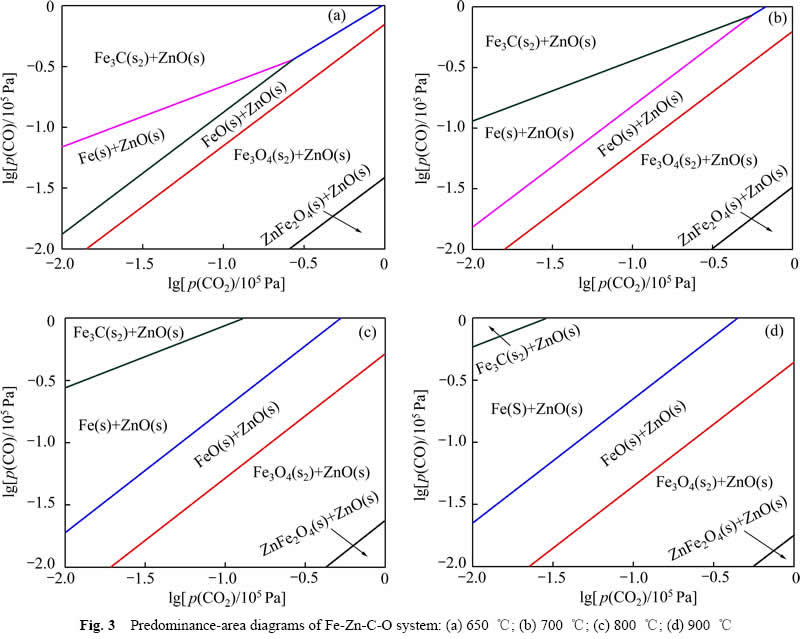
图3 Fe-Zn-C-O系含锌物种的优势区域图
由图4(a)可知,随着焙烧气氛中CO浓度从1%增加至9%,铁酸锌的分解率从48.2%逐渐增加至65.3%和产物的w(Fe2+)/w(FeT)值从0.26逐渐上升至0.53。当CO浓度为3%时,焙烧产物的w(Fe2+)/w(FeT)值约为1/3,当CO浓度超过3%后,焙烧产物中的部分铁物相就会被过还原为氧化亚铁,而铁酸锌的分解率一直保持在66%以内,这说明在还原焙烧过程中,反应(2)和反应(3)会伴随反应(1)发生,再结合图2可知,在该条件下造成铁物相过还原的主要原因是反应(2)的发生。当CO浓度增至5%时,铁酸锌的分解率达到60%,此时w(Fe2+)/w(FeT)值约为0.4,略微超过了理想值1/3。因此,综合考虑,CO浓度为5%时还原焙烧效果较佳。从图4(b)可以看出,还原气氛中CO与CO+CO2的分压比对铁酸锌的分解行为影响更大。当p(CO)/p(CO+CO2)值在0.1以内,随着CO分压的增加,铁酸锌的分解率迅速增加,当p(CO)/p(CO+CO2)超过0.1以后,铁酸锌分解率的增加速度变缓。对于Fe2+的含量而言,随着CO的分压不断增加,产物中Fe2+含量先缓慢增加,当p(CO)/p(CO+CO2)超过0.2以后,Fe2+含量的增加速度加快。由此可见,p(CO)/p(CO+CO2)低于0.2的还原气氛有利于抑制铁酸锌的过还原。由图4(c)可知,还原气体流量对铁酸锌的分解效果有一定的影响。当气体流量低于1 L/min时,铁酸锌的分解率和产物的w(Fe2+)/w(FeT)值随着气体流量的增加而增加,当气体流量大于1 L/min后,这两个指标不再随着气体流量的增加而上升,表明当气体流量为1 L/min时就能提供足够的还原气氛,继续增加气量不会进一步改变还原焙烧气氛的组成,因此对铁酸锌的分解行为基本不再造成影响。
除了还原气氛外,焙烧温度也是影响还原反应的重要因素。由图4(d)可知,温度对铁酸锌的分解行为影响较大。随着焙烧温度从500 ℃增加至900 ℃,铁酸锌的分解率从40.9%逐渐增至68.8%,产物的w(Fe2+)/w(FeT)值从0.31逐渐增加到0.57。当焙烧温度在700 ℃以内,随着焙烧温度的增加铁酸锌的分解率迅速增加,而焙烧产物的w(Fe2+)/w(FeT)值缓慢增加;当温度超过700 ℃以后,随着焙烧温度的增加铁酸锌的分解率增加速度变缓,而焙烧产物的w(Fe2+)/w(FeT)值上升速度加快。由此可知,在相对低温下铁酸锌的还原反应主要以反应(1)的形式进行,升高温度可以显著促进反应(1)的正向进行;当温度高于700 ℃后,控制反应(1)进行的主要因素逐渐从温度转变为其他条件,而反应(2)和反应(3)因温度的增加明显加快,导致Fe2+含量开始迅速增加,这与第2.1节热力学分析结果一致。从图4(e)可以看出,回转窑转速对铁酸锌分解行为的影响与还原气体流量的影响相似,当转速达到5 r/min后,回转窑中样品的翻动效果达到最佳,继续增加转速不仅不能进一步促进铁酸锌的分解还会造成能源浪费。由图4(f)可以看出,焙烧时间对铁酸锌的分解行为也有重要的影响。随着时间从15 min增至60 min,铁酸锌的分解率从39.9%迅速增加至60.2%,产物的w(Fe2+)/w(FeT)值从0.28上升到0.40;随着反应的继续进行,铁酸锌的分解率在120 min时可增加至69.1%,而Fe2+的含量的增加速度在1 h后变缓。因此,延长焙烧时间有利于铁酸锌的分解,但是时间过长会造成能耗的加大和焙烧效率的下降,故焙烧时间不宜超过2 h。

图4 焙烧条件对铁酸锌分解行为的影响
总体而言,上述考察的因素都会影响铁酸锌的分解行为,CO浓度、p(CO)/p(CO+CO2)值、焙烧温度和时间是影响铁酸锌的分解主要原因,气体流量和回转窑转速只在一定范围内对铁酸锌的分解行为有较大的影响。根据以上分析可得,使锌焙砂中铁酸锌选择性的分解为氧化锌和四氧化三铁的优化条件如下:CO浓度5%、p(CO)/p(CO+CO2)=0.2、气体流量1 L/min和焙烧温度700 ℃、回转窑转速5 r/min及焙烧时间2 h,在此条件下铁酸锌的分解率近70%,且铁物相的过还原不严重。
2.3 还原焙烧过程矿物学特征变化研究
为了进一步研究还原焙烧过程中铁酸锌的分解行为,采用XRD考察了不同焙烧时间下产物的晶体物相特征,其结果如图5所示。由图5可知,焙烧产物中所含的主要物相为ZnO、Fe3O4、ZnS和Zn2SiO4。结合第1.1节的讨论可知,未经还原焙烧样品中的铁物相主要以铁酸锌形式存在。当还原焙烧时间为30 min时,ZnFe2O4特征峰略有降低,同时四氧化三铁特征峰略有增加,表明此时仅有小部分铁酸锌转化为四氧化三铁。当焙烧时间达到60 min时,ZnFe2O4特征峰不仅进一步降低而且还向右偏移,同时铁酸锌的峰明显增加。ZnFe2O4特征峰右移的主要原因是在反应过程中ZnFe2O4分子结构中的部分Zn2+被Fe2+取代,且Fe2+离子半径小于Zn2+离子半径,导致铁酸锌的晶距变小,从而使ZnFe2O4特征峰右移。当焙烧时间为120 min时,锌焙砂中的大部分铁酸锌转化为四氧化三铁,所以延长焙烧时间有利于将锌焙砂中的铁酸锌选择性的转化为氧化锌和四氧化三铁。
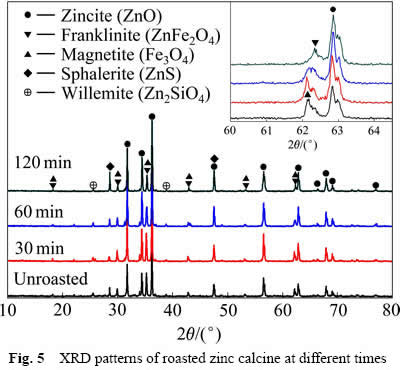
图5 不同时间下焙烧产物的XRD谱
为了考察还原焙烧产物的微观形貌特征及其组成成分,试验采用SEM和EDS对焙烧时间为2h的产物进行了研究,其结果如图6所示。从图6可以看出,焙烧产物的粒度较小,基本在20 μm以下,矿物颗粒疏松多孔,物相间互相包裹严重,未出现明显的烧结现象,因此,在后续试验中很难通过“磨矿-磁选”的方法实现锌铁分离,宜采用低酸浸出的方法进行锌铁分离。经EDS鉴定,图6(a)中表面致密光滑的灰色物相(A)主要含有Zn和O,还有少量的Fe和微量的Mn,说明该物相是夹杂有少量的Fe3O4和Mn杂质的ZnO;图中表面相对粗糙的白色物相(B)主要含有Zn和O,还有一定量的Fe和S元素,表明这部分物相可能是ZnO、Fe3O4和ZnS的混合物。
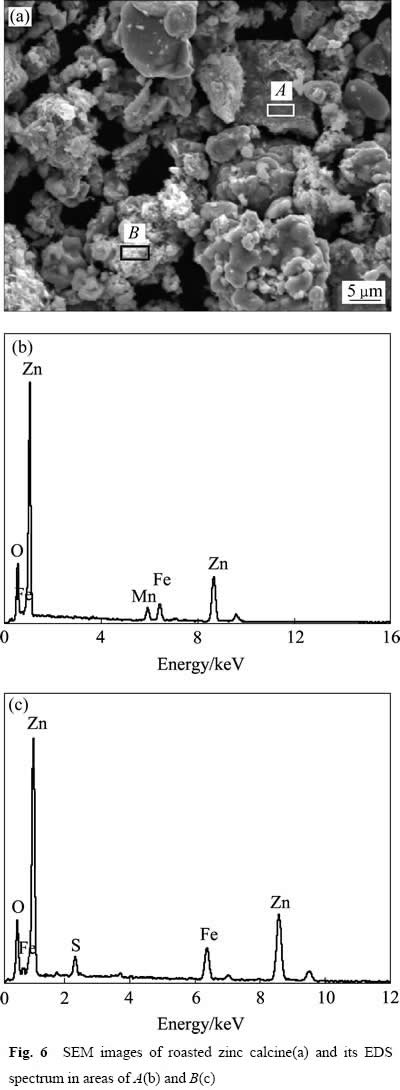
图6 还原焙烧产物的SEM像及EDS谱
3 结论
1) 根据热力学分析可知,CO能将ZnFe2O4还原为ZnO和Fe3O4,但也易过还原为FeO。反应温度及CO和CO2的分压对铁酸锌还原优势区域影响较大,提高温度、增加CO分压和降低CO2分压会促进ZnFe2O4的分解,同时也会增加FeO和Fe的优势区域,在适宜的温度和气氛下,可以实现铁酸锌的选择性分解。
2) 通过还原焙烧可以将锌焙砂中大部分的ZnFe2O4选择性分解为ZnO和Fe3O4。CO浓度、p(CO)/p(CO+CO2)值、焙烧温度和时间是影响铁酸锌分解行为的主要原因,还原气体流量和回转窑转速只在一定范围内对铁酸锌的分解行为有较大的影响。最优焙烧条件为:CO浓度5%、p(CO)/p(CO+CO2)=0.2、气体流量1 L/min和焙烧温度700 ℃、回转窑转速5 r/min及焙烧时间2 h,在此条件下铁酸锌的分解率近70%,且铁物相的过还原现象不严重。
3) 由XRD分析可知,焙烧产物主要以ZnO、Fe3O4、ZnS和Zn2SiO4物相为主,ZnFe2O4被逐渐还原为ZnO和Fe3O4。经SEM和EDS分析,还原样品颗粒疏松多孔,粒度较小,互相包裹严重,未出现明显的烧结现象,因此,在后续试验中很难通过磁选的方法实现锌铁分离,应采用低酸浸出的方法进行锌铁分离。
REFERENCES
[1] BALARINI J C, LUDMILA de O P, MIRANDA T L S, CASTRO R M Z d, SALUM, A. Importance of roasted sulphide concentrates characterization in the hydrometallurgical extraction of zinc[J]. Minerals Engineering, 2008, 21(1): 100-110.
[2] SANTOS S M C, MACHADO R M, CORREIA M J N, REIS M T A, ISMAEL M R C, CARVALHO J M R. Ferric sulphate/chloride leaching of zinc and minor elements from a sphalerite concentrate[J]. Minerals Engineering, 2010, 23(8): 606-615.
[3] ?OPUR M, ?ZMETIN C, ?ZMETIN E, KOCAKERIM M M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions[J]. Chemical Engineering and Processing: Process Intensification, 2004, 43(8): 1007-1014.
[4] 彭海良. 常规湿法炼锌中铁酸锌的行为研究[J]. 湖南有色金属, 2004, 20(5): 20-22.
PENG Hai-liang. Study on the behavior of zinc ferrite in conventional hydrometallurgical zinc production process[J]. Hunan Nonferrous Metals, 2004, 20(5): 20-22.
[5] CHEN T, DUTRIZAC J. Mineralogical changes occurring during the fluid-bed roasting of zinc sulfide concentrates[J]. JOM, 2004, 56(12): 46-51.
[6] JHA M K, KUMAR V, SINGH R J. Review of hydrometallurgical recovery of zinc from industrial wastes[J]. Resources, Conservation and Recycling, 2001, 33(1): 1-22.
[7] ERDEM M, ?ZVERDI A. Environmental risk assessment and stabilization/solidification of zinc extraction residue: Ⅱ. Stabilization/solidification [J]. Hydrometallurgy, 2011, 105(3): 270-276.
[8] TURAN M D, ALTUNDOGAN H S, TUMEN F. Recovery of zinc and lead from zinc plant residue[J]. Hydrometallurgy, 2004, 75(1/4): 169-176.
[9] ?OPUR M, PEKDEMIR T, ?OLAK S, KUNKUL A. Industrial symbiosis: High purity recovery of metals from Waelz sintering waste by aqueous SO2 solution[J]. Journal of Hazardous Materials, 2007, 149(2): 303-309.
[10] 杨 斌. 对湿法炼锌中热酸浸出-黄钾铁矾工艺的探讨[J]. 甘肃冶金, 2010, 32(3): 56-58.
YANG Bin. Discussion on hot acid leach-jarosite process of hydrometallurgy zinc[J]. Gansu metallurgy, 2010, 32(3): 56-58.
[11] WANG Xin, SNINIVASAKANNAN C, DUAN Xin-hui, PENG Jin-hui, YANG Da-jin, JU shao-hua. Leaching kinetics of zinc residues augmented with ultrasound[J]. Separation and Purification Technology, 2013, 115: 66-72.
[12] JU Shao-hua, ZHANG Yi-fei, ZHANG Yi, XUE Pei-yi, WANG Yi-hui. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy[J]. Journal of Hazardous Materials, 2011, 192(2): 554-558.
[13] 邓永贵, 陈启元, 尹周澜, 张平民. 锌浸出液针铁矿法除铁[J]. 有色金属(冶炼部分), 2010, 62(3): 80-84.
DENG Yong-gui, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min. Removal of ferrous/ferric iron from zinc leaching solution by goethite process[J]. Nonferrous Metals (Extractive Metallurgy), 2010, 62(3): 80-84.
[14] 赵 永, 蒋开喜, 王德全, 郭亚会. 用针铁矿法从锌焙烧烟尘的热酸浸出液中除铁[J]. 有色金属(冶炼部分), 2005(5): 13-15.
ZHAO Yong, JIANG Kai-xi, WANG De-quan, GUO Ya-hui. Iron removal from hot acid Leaching solution of zinc roasting dust by goethite process[J]. Nonferrous Metals (Extractive Metallurgy), 2005(5): 13-15.
[15] 岳 明, 孙宁磊, 邹 兴, 邵建春, 刘金山, 王魁珽, 陆业大. 锌浸出液三价铁直接水解赤铁矿法除铁的探讨[J]. 中国有色冶金, 2012, 41(4): 80-85.
YUE Ming, SUN Ning-lei, ZOU Xing, SHAO Jian-chun, LIU Jin-shan, WANG Kui-ting, LU Ye-da. The discussion on hydrolysis precipitation of ferric oxide directly from ferric-iron rich zinc leachate[J]. China Nonferrous Metallurgy, 2012, 41(4): 80-85.
[16] HOLLOWAY P C, ETSELL T H, MURLAND A L. Roasting of La oroya zinc ferrite with Na2CO3[J]. Metallurgical and Materials Transactions B, 2007, 38(5): 781-791.
[17] HOLLOWAY P C, ETSELL T H, MURLAND A L. Use of secondary additives to control the dissolution of iron during Na2CO3 roasting of La oroya zinc ferrite[J]. Metallurgical and Materials Transactions B, 2007, 38(5): 793-808.
[18] ZHANG Ya-li, YU Xian-jin, LI Xiao-bin. Zinc recovery from franklinite by sulphation roasting[J]. Hydrometallurgy, 2011, 109(3): 211-214.
[19] 王纪明, 彭 兵, 柴立元, 李 密, 彭 宁. 锌浸渣还原焙 烧-磁选回收铁[J]. 中国有色金属学报, 2012, 22(5): 1455-1461.
WANG Ji-ming, PENG Bing, CHAI Li-yuan, LI Mi, PENG Ning. Recovery iron from zinc leaching residues by reduction roasting and magnetic separation process[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(5): 1455-1461.
[20] LI Min, PENG Bing, CHAI Li-yuan, WANG Ji-ming, PENG Ninf, YAN Huan. Element distribution of high iron-bearing zinc calcine in high gradient magnetic field[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2261-2267.
[21] YAN Huan, CHAI Li-yuan, PENG BING, LI Mi, PENG Ning, HOU Dong-ke. A novel method to recover zinc and iron from zinc leaching residue[J]. Minerals Engineering, 2014, 55: 103-110.
[22] LIU Wei, HAN Jun-wei, QIN Wen-qing, CHAI Li-yuan, HOU Dong-ke, KONG Yan. Reduction roasting of high iron-bearing zinc calcine for recovery of zinc and iron[J]. Canadian Metallurgical Quarterly, 2014, 53(2): 176-182.
[23] 韩俊伟, 刘 维, 覃文庆, 柴立元, 郑永兴, 杨 康. 高铁锌焙砂选择性还原焙烧-两段浸出锌[J]. 中国有色金属学报, 2014, 24(2): 511-518.
HAN Jun-wei, LIU Wei, QIN Wen-qing, CHAI Li-yuan, ZHENG Yong-xing, YANG Kang. Leaching zinc from high iron-bearing zinc calcine after selective reduction roasting[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(2): 511-518.
Selective decomposition behavior of zinc ferrite by reduction roasting with CO
HAN Jun-wei1, LIU Wei1, QIN Wen-qing1, CAI Xin2, LUO Hong-lin1, WANG Da-wei1
(1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: In order to selectively decompose ZnFe2O4 contained in zinc calcine to ZnO and Fe3O4, the decomposition behavior of zinc ferrite during CO reduction roasting process was studied. The thermodynamic basis of ZnFe2O4 decomposition with CO was firstly calculated by HSC and Factsage software, then the effects of reduction roasting conditions on ZnFe2O4 decomposition behavior were investigated by reduction roasting test using rotary kiln. The results show that ZnFe2O4 can be selectively converted into ZnO and Fe3O4 at appropriate temperature and atmosphere. CO concentration, p(CO)/p(CO+CO2), roasting temperature and time have significant effects on the selective decomposition behavior, and increasing them are conducive to the decomposition of ZnFe2O4 and the generation of FeO. Under the optimum condition, the decomposition rate of ZnFe2O4 is nearly 70% without substantive over-reduction of iron. The roasting product mainly composes of ZnO, Fe3O4, ZnS and Zn2SiO4, and the particles are small, loose, porous and seriously mutual encapsulated according to XRD and SEM / EDS analyses.
Key words: zinc ferrite; zinc calcine; zinc residue; reduction roasting; decomposition behavior
Foundation item: Project(51204210) supported by the National Natural Science Foundation of China; Project (2012BAC12B04) supported by the National Science and Technology Pillar Program during the 12th “Five-year” Plan Period; Project(2011AA061001) supported by the National High Research Development Program of China
Received date: 2015-01-30; Accepted date: 2016-03-10
Corresponding author: LIU Wei; Tel: +86-731-88830884; E-mail: ase.6520@163.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51204210);国家“十二五”科技支撑计划资助项目(2012BAC12B04);国家高技术研究发展计划资助项目(2011AA061001)
收稿日期:2015-01-30;修订日期:2016-03-10
通信作者:刘 维,讲师,博士;电话:0731-88830884;E-mail: ase.6520@163.com