硫化法除钼过程中杂质砷的行为
谢昊1,2,赵中伟1,2,曹才放1,2,梁勇1,2,李江涛1,2
(1. 中南大学 冶金科学与工程学院,湖南 长沙,410083;
2. 中南大学 稀有金属与材料制备湖南省重点实验室,湖南 长沙,410083)
摘要:根据已有的热力学数据,对钨钼硫化分离过程中杂质砷的各种配合物进行计算,绘制溶液中各含砷离子随pH及S与As的浓度比[S]/[As]变化的分布曲线,分析溶液中pH及硫化剂用量对砷硫化行为的影响规律。研究结果表明:控制一定的条件,将溶液中的含砷化合物较彻底地硫化为AsS43-在热力学上是完全可能的;溶液的pH是影响硫化效果的关键因素,并且砷的硫化程度随硫化剂用量的增加而增大;在pH=7.5~9.5范围内,硫用量 [S]/[As]>6时可使99%以上的As硫化为AsS43-。
关键词:硫代砷酸盐;除砷;硫化反应;热力学
中图分类号:TF841.1 文献标志码:A 文章编号:1672-7207(2012)02-0435-05
Behavior of arsenic in process of removing molybdenum by sulfide method
XIE Hao1,2, ZHAO Zhong-wei1,2, CAO Cai-fang1,2, LIANG Yong1,2, LI Jiang-tao1,2
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Hunan Key Laboratory for Metallurgy and Material Processing of Rare Metals,Central South University, Changsha 410083, China)
Abstract: According to the known thermodynamic data, the sulfidizing behavior of arsenic complexes in the sulfidizing separation of tungsten and molybdenum was calculated, and the distribution graphs of various arsenic ions vs pH and arsenic ion vs molar ratio of S to As were drawn. The influence law of pH and sulfidizing agent dosage on sulfidizing behavior of arsenic were discussed. The results show that, under certain conditions, the arsenic sulfide compounds can thoroughly be transformed into AsS43- in thermodynamics. The pH value of the solution is the key factor in sulfidizing effect, and the extent of arsenic sulfidizing increases with the increase of the sulfidizing agent dosage. More than 99% of As can be sulfidized to AsS43- under the conditions of pH 7.5-9.5 and mole ratio of S to As above 6.
Key words: thio-arsenate; removal of arsenic; sulfidation reaction; thermodynamics
随着钨矿资源的不断开发和利用,优质钨资源日益减少,处理品位低、杂质含量高的钨矿已成为钨冶金中一个很现实的问题。这些高杂质的钨矿中,除了杂质元素钼以外,砷的含量也日益增加。而随着硬质合金、电子、国防等工业的发展,对钨制品尤其是对许多尖端用途所需的钨制品中的杂质含量要求越来越严格。在钨冶金产品中,砷是需严格控制的杂质元素,如国标GB 10116—88中规定在APT-0级产品中,杂质砷的质量分数要小于10×10-6。钨酸钠溶液中净化除砷的一些方法,有经典沉淀净化法[1-2]、强碱性阴离子树脂交换法[3]、离子交换法、离子浮选法和溶剂萃取法[4-5]等。但采用这些方法要么钨损失较大,渣量大,要么流程冗长,设备复杂。选择性沉淀法在钨酸盐溶液中除钼已经被广泛采用[6]。工业上应用这一新工艺时,一部分杂质砷也会在除钼过程中除去,说明砷酸根也可能硫化成为了硫代砷酸盐[7-8],进而也像硫代钼酸根一样被结合。进行杂质砷的硫化行为的研究对于进一步优化条件,提高砷的硫化转化率及沉淀脱除率,从而实现钼、砷的同时深度脱除具有重要 意义。
1 热力学计算及计算方法
在As-S-H2O体系中,存在表1所示的平衡关系式。所有数据均引自文献[9]。其中:[H3AsSO3]表示H3AsSO3的浓度,mol/L。其余含义依此类推。
本研究需考虑同时存在3价砷化合物和5价砷化合物的溶液体系。因此,需要在3价砷和5价砷之间建立氧化还原的关系式。这里选择如下的关系式(由于各含砷离子的活度系数参数尚无文献报道,并且对高浓度溶液的热力学计算尚无有效解决办法,故在计算中均以浓度代替活度):
H3AsO4+H2S(aq)=H3AsO3+S+H2O (32)
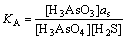
2AsS+S=As2S3 (33)
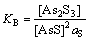
式中:KB=3.9。由式(33)得出as= KB-1。由于式(32)中各组分的自由能已知,得到KA=14.47。
表1 25 ℃时As-S-H2O体系的平衡反应及其平衡常数
Table 1 Equilibrium reactions and constants for As-S-H2O system at 25 ℃

由(1)~(33)平衡关系式可以看出:溶液中游离的As和S的游离形态有H2S(aq),HS-,H3AsO3,H2AsO3-,HAsO32-,H3AsSO2,H2AsSO2-,HAsSO22-,H3AsS2O,H2AsS2O-,HAsS2O2-,H3AsS3,H2AsS3-,HAsS32-,H3AsO4,H2AsO4-,HAsO42-,AsO43-,H3AsSO3,H2AsSO3-,HAsSO32-,AsSO33-,H3AsS2O2,H2AsS2O2-,HAsS2O22-,AsS2O23-,H3AsS3O,H2AsS3O-,HAsS3O2-,AsS3O3-,H3AsS4,H2AsS4-,HAsS42-,AsS43-。
各含S溶解组分浓度总和等于总S浓度:
[S]=[H2S]+[HS-]+[H3AsSO2]+[H2AsSO2-]+
[HAsSO22-]+2[H3AsS2O]+2[H2AsS2O-]+
2[HAsS2O2-]+3[H3AsS3]+3[H2AsS3-]+3[HAsS32-]+
[H3AsSO3]+[H2AsSO3-]+[HAsSO32-]+[AsSO33-]+
2[H3AsS2O2]+2[H2AsS2O2-]+2[HAsS2O22-]+
2[AsS2O23-]+3[H3AsS3O]+3[H2AsS3O-]+
3[HAsS3O2-]+3[AsS3O3-]+4[H3AsS4]+
4[H2AsS4-]+4[HAsS42-]+4[AsS43-]
各含As溶解组分浓度总和等于总As浓度:
[As]=[H3AsO3]+[H2AsO3-]+[HAsO32-]+[H3AsSO2]+
[H2AsSO2-]+[HAsSO22-]+[H3AsS2O]+[H2AsS2O-]+
[HAsS2O2-]+[H3AsS3]+[H2AsS3-]+[HAsS32-]+
[H3AsO4]+[H2AsO4-]+[HAsO42-]+[AsO43-]+
[H3AsSO3]+[H2AsSO3-]+[HAsSO32-]+[AsSO33-]+
[H3AsS2O2]+[H2AsS2O2-]+[HAsS2O22-]+[AsS2O23-]+
[H3AsS3O]+[H2AsS3O-]+[HAsS3O2-]+[AsS3O3-]+
[H3AsS4]+[H2AsS4-]+[HAsS42-]+[AsS43-]
2 结果与讨论
2.1 pH对硫化行为的影响
若给定砷、硫的浓度及体系的pH,联立(32),(33)式以及表1中(1)~(31)的浓度关系式,可得到1个含有33个未知数的方程组,解这个方程组可得各含砷离子的浓度。
砷在低pH环境下,会形成As2S3的沉淀,存在溶解平衡;在高pH环境下,砷会形成各种含砷的离子。因此,需要分段计算。
除砷过程是在钨酸盐溶液中除钼的情况下进行,因此,参考钨钼分离的常用工业条件,取[S]=0.15 mol/L[10],假定溶液中含[As]=0.0015 mol/L[11-12],考察各含砷离子分布随pH变化的情况,结果见图1。
从图1可知:当pH为2~5时,溶液中砷的溶解度随着pH的减小而减小;当pH=2时,溶液中总砷浓度仅为8.5×10-5 mol/L,As2S3的沉淀率达到94.3%;在pH=4~6时,H2AsS3-是溶液中的主要离子;在pH>6时,溶液中5价砷的化合物AsS43-成为溶液中最主要的阴离子,并且在pH=7~12时,AsS43-浓度曲线和总砷浓度曲线重合,As全部被硫化为AsS43-。
在工业上除钼的条件下,从热力学上分析,溶液中的砷可以被完全硫化形成AsS43-。
2.2 硫用量对砷硫化行为的影响
取总砷量[As]=0.0015 mo1/L,pH分别取7.5,8.5和9.5,针对不同的硫用量[S]计算各含砷离子的平衡浓度分数,以考察硫加入量与砷硫化效果的关系,结果见图2~4(图中只列出了主要离子,其余离子由于含量过低而无法正常显示)。图2~4中:[As]表示As的浓度,mol/L;[As]T表示As的总浓度,mol/L。
由图2~4可知:在溶液pH=7.5,硫用量[S]/[As]=4时(即理论用量),完全硫化的AsS43-只占90%,HAsS3O2-占8.9%,这显然不满足要求;当硫用量[S]/[As]=6时,已可使99 %的砷以AsS43-的形式存在,这相当于溶液中游离硫离子的浓度约为0.003 mol/L;再进一步增加硫用量,对硫化的促进作用渐弱;当加大硫用量至[S]/[As]=20时,也只能使完全硫化率增至99.4%,此时,溶液中游离硫离子浓度约为23 mmol/L。
在溶液pH=8.5,硫用量[S]/[As]=4时,砷的完全硫化率为91.8%;当硫用量[S]/[As]=6时,砷的完全硫化率为99.6%。
在溶液pH=9.5,硫用量[S]/[As]=4时,砷的完全硫化率为92%;当硫用量[S]/[As]=6时,砷的完全硫化率为99.6%。
从以上分析可以看到:AsS43-的平衡分布随硫浓度的增加而急剧增加,而当在硫砷比为5时,其他的硫代砷酸根离子几乎都不存在。对比图1~4可看到:当碱性溶液pH<11时,As几乎全部以AsS43-存在于水溶液中;当溶液pH<5时,As出现沉淀。对比钼在钨钼分离过程中发生的反应[13],理论上,砷完全硫化所需的硫用量更低,更容易被硫化成为硫代砷酸根。
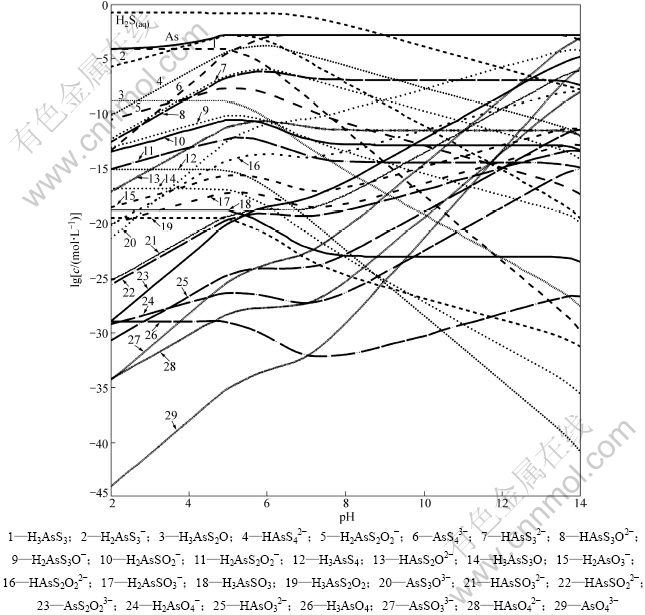
图1 各含砷离子分布随pH变化的分布曲线
Fig.1 lgc-pH curves for different arsenic ions at 25 ℃
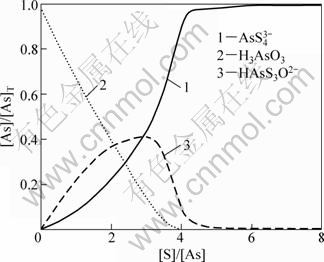
图2 pH=7.5时硫浓度对硫化效果的影响
Fig.2 Influences of sulfur dosage on effect of sulfidation at pH=7.5
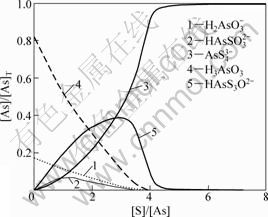
图3 pH=8.5时硫浓度对硫化效果的影响
Fig.3 Influences of sulfur dosage on effect of sulfidation at pH=8.5
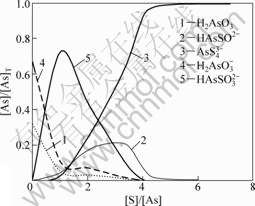
图4 pH=9.5时硫浓度对硫化效果的影响
Fig.4 Influences of sulfur dosage on effect of sulfidation at pH=9.5
3 结论
(1) 控制一定的条件,将溶液中的含砷化合物较彻底地硫化为AsS43-在热力学上是完全可能的。
(2) 溶液的pH是影响硫化效果的关键因素,As的硫化程度随pH的增大有峰值。
(3) 砷的硫化程度随硫用量的增加而增大,但当硫用量增加到一定程度后,进一步增加硫用量对硫化的促进作用并不显著;在溶液pH减小时,为了取得较好的硫化效果,需相应地增加硫化剂的用量。
(4) 在pH=7.5~9.5范围内,在硫用量[S]/[As]>6时,可使99%以上的As硫化为AsS43-。
参考文献:
[1] 李洪桂. 稀有金属冶金学[M]. 北京: 冶金工业出版社, 1990: 55-56.
LI Hong-gui. Rare metals metallurgy[M]. Beijing: Metallurgical Industry Press, 1990: 55-56.
[2] 李洪桂. 有色金属提取冶金手册:稀有高熔点金属: 上卷(W, Mo, Re, Ti)[M]. 北京: 冶金工业出版社, 1999:118-119.
LI Hong-gui. Non-ferrous metal extraction metallurgy handbook: Rare refractory metals: Vol. 1(W, Mo, Re, Ti)[M]. Beijing: Metallurgical Industry Press, 1999:118-119.
[3] 陈洲溪, 黄芍英, 周良益, 等. 离子交换法分离钨酸盐溶液中的钼: 中国, 881057126[P]. 1988-05-16.
CHEN Zhou-xi, HUANG Shao-ying, ZHOU Liang-yi, et al. Removing molybdenum from tungstate solution by using ion exchange technics: CN 881057126[P]. 1988-05-16.
[4] 黄蔚庄, 龚柏凡, 张启修. 溶剂萃取硫代钼酸盐分离钨钼[J]. 中国有色金属学报, 1995, 5(1): 45-47.
HUANG Wei-zhuang, GONG Bai-fan, ZHANG Qi-xiu. Separation of Tungsten and molybdenum by solvent extraction with thio molybdate[J]. The Chinese Journal of Nonferrous Metals, 1995, 5(1): 45-47.
[5] Zheng Q Y, Fan H H. Separation of molybdenum from tungsten by di-2-ethylhexyl phosphoric acid ext reactant[J]. Hydrometallurgy, 1986, 16(3): 263-270.
[6] 孙培梅, 李洪桂, 李运姣, 等. 选择性沉淀法从钨酸盐溶液中除钼的工业试验[J]. 中国有色金属学报, 2001, 11(3): 499-502.
SUN Pei-mei, LI Hong-gui, LI Yun-jiao, et al. Commercial scale test of selective precipitation method for Mo removal from tungstate solution[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(3): 499-502.
[7] 肖若珀. 砷的提取、环保和应用方向[M]. 柳州: 广西金属学会出版社,1992: 58.
XIAO Ruo-po. Extraction, environmental protection and application direction of arsenic[M]. Liuzhou: Guangxi Society for Metals Press, 1992: 58.
[8] 张启修, 肖连生, 龚柏凡. 一步离子交换法生产APT新工艺的沿革与现状[J]. 中国钨业, 2000, 15(1): 24-25.
ZHANG Qi-xiu, XIAO Lian-sheng, GONG Bai-fan. The developmental curse and status of producing APT with oe-step in a change process[J]. China Tungsten Industry, 2000, 15(1): 24-25.
[9] Helz G R, Tossell J A. Thermodynamic model for arsenic speciation in sulfidic waters: A novel use of ab initio computations[J]. Geochimica et Cosmochimica Acta, 2008, 72(18): 4457-4468.
[10] 霍广生, 赵中伟, 吴保林. 钼的硫化反应热力学分析[J]. 中南工业大学学报: 自然科学版, 2001, 32(3): 259-261.
HUO Guang-sheng, ZHAO Zhong-wei, WU Bao-lin. Thermal dynamic analysis on sulfidation of molybdate[J]. Journal of Central South University of Technology: Natural Science, 2001, 32(3): 259-261.
[11] 孙培梅, 陈洲溪, 陈树翘, 等. 用钨细泥制取钨酸钠和仲钨酸铵新工艺研究[J]. 中国钨业, 1993(4): 10-15.
SUN Pei-mei, CHEN Zhou-xi, CHEN Shu-qiao, et al. Study on new technology of producting sodium tungstate and APT with tungsten fine deposit[J]. China Tungsten Industry, 1993(4): 10-15.
[12] 刘茂盛, 孙培梅, 李运姣, 等. 关于钨冶炼离子交换工艺除杂效果的探讨[J]. 稀有金属与硬质合金, 1990, 101: 8-15.
LIU Mao-sheng, SUN Pei-mei, LI Yun-jiao, et al. Study ion exchange process on the tungsten smelting effects of impurities[J]. Rare Metals and Cemented Carbides, 1990, 101: 8-15.
[13] 卢江波, 赵中伟, 李洪桂. 钨钼的硫化反应热力学分析[J]. 中国钨业, 2005, 20(4): 33-38.
LU Jiang-bo, ZHAO Zhong-wei, LI Hong-gui. Thermodynamic analysis on sulfidation of molybdate and tungstate[J]. China Tungsten Industry, 2005, 20(4): 33-38.
(编辑 陈灿华)收稿日期:2011-03-15;修回日期:2011-06-02
基金项目:国家高技术研究发展计划(“863”计划)项目(2007AA06Z129)
通信作者:赵中伟(1966-),男,河北永年人,教授,从事冶金及功能材料的研究;电话:0731-88830476;E-mail:zhaozw@csu.edu.cn