文章编号:1004-0609(2007)08-1379-06
电极指示法在硫酸锌浸出液脱氯反应中的应用
王松森,李元高,曾孟祥,陶 涛,张平民
(中南大学 化学化工学院,长沙 410083)
摘 要:根据Cu-Cl?-H2O多相体系的φ—pH图和φ—lga(Cl?)图,采用CuCl沉淀法脱去硫酸锌浸出液中的杂质Cl?,在加锌粉过程中,分别采用铜丝电极和铂片电极为指示电极连续测定φ (Cu2+/Cu)与φ[Cu2+/CuCl(s)],并讨论了浸出液中Cu2+含量对除氯的影响。结果表明:当φ(Cu2+/Cu)与φ[Cu2+/CuCl(s)]首次相等时,体系中金属铜、Cu2+和固态CuCl建立三相平衡,此时除氯效果最佳;除氯效果随Cu2+含量的增加而提高;Cu2+含量大于0.15 mol/L才能达到除氯要求(Cl?含量小于300 mg/L);Cu2+含量增加到0.20 mol/L以后,除氯效果没有明显提高。
关键词:CuCl;脱氯;Cu-Cl?-H2O体系;指示电极
中图分类号:O646.2 文献标识码:A
Application of indicator electrodes in chloride removal from ZnSO4 leachate
WANG Song-sen, LI Yuan-gao, ZENG Meng-xiang, TAO Tao, ZHANG Ping-min
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: Based on the potential—pH diagram and potential—lga(Cl?) diagram of Cu-Cl?-H2O multiphase system, in the chloride removal process by cuprous chloride precipitation copper indictor electrode and platinum indictor electrode were used to continually monitor the φ(Cu2+/Cu) and φ[Cu2+/CuCl(s)], respectively. The effect of Cu2+ content on the chloride removal effect was researched. The results show that when the two potentials intersect at the same point for the first time, which means that the equilibrium is established at the triple point, the effect of optimum chloride removal can be gained. The final Cl? content can be decreased to 300 mg/L when Cu2+ content is 0.15 mol/L, and the effect of chloride removal can be improved as Cu2+ content increases, but when Cu2+ content is more than 0.20 mol/L, the effect of chloride removal cannot be obviously improved any more.
Key words: cuprous chloride; chloride removal; Cu-Cl?-H2O system; indictor electrodes
在现代湿法炼锌过程中,由于处理的锌焙砂、各种烟尘、氧化锌粉以及其他含锌物料(如铸型渣等)中都含有一定量的氯,这些物料中的氯在浸出过程中,几乎全部进入溶液。氯的存在影响锌电解沉积过程,如使阳极和冷却器腐蚀,电解液含铅升高,使阴极析出锌质量降低等。为此,当硫酸锌浸出液中Cl?含量大于300 mg/L时,就应该设法净化除氯[1?2]。除氯的方法很多,工业上常采用的是硫酸银沉淀法、铜渣除氯法、离子交换法[3?5]。
李春[6]、李岚[7]和Zabaleta[8]研究了采用CuCl沉淀法进行脱氯,向已溶有一定量硫酸铜的硫酸锌浸出液中加入锌粉,生成活性很强的铜单质,在一定pH和电位范围内,铜单质可与Cu2+子发生氧化还原反应生成Cu+,并立即又与Cl?作用生成沉淀CuCl(s),能使Cl?含量降低到300 mg/L以下,可满足电解要求。采用该方法成本低,除氯效果好,效率高,不会引进其它杂质。然而,采用这种除氯方法,还原剂锌粉的加入量难以准确控制,同时锌粉量对除氯效果影响很大:当锌粉加入量少时,Cl?脱除达不到要求;当锌粉过量时,它能继续将Cu+还原为铜单质,CuCl(s)发生返溶,使电解液中Cl?含量升高。
本文作者根据Cu-Cl?-H2O多相体系的φ—pH图和φ—lga(Cl?)图,采用CuCl沉淀法脱去硫酸锌浸出液中的杂质氯离子,以铜丝和铂片电极为双电极指示,根据指示电位的变化情况控制锌粉的加入量,在φ—lga(Cl?)图中特定点上,使体系中金属Cu、Cu2+和固态CuCl建立三相平衡时,达到除氯的最大限度。
1 实验原理
在含氯的硫酸锌浸出液中加入一定量的五水硫酸铜(CuSO4?5H2O)并溶解,然后加入锌粉,发生的反应为:

由于新生成的铜活性很强,在一定的pH和电位范围内,可与Cu2+发生氧化还原反应生成Cu+,又立即与Cl?作用生成沉淀CuCl(s),从而将溶液中的杂质氯除去,反应为
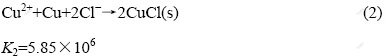
由于锌粉还原能力很强,当加入的锌粉过量时,它能继续将Cu+还原为Cu单质,即CuCl(s)发生返溶。反应为
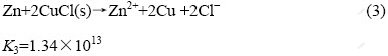
因此,还原剂锌粉的加入量对除氯效果影响很大。
图1所示为Cu-Cl?-H2O体系的φ—pH图[9]。由图1可知,在标准状态下,当a(Cl?)=1 mol/L,a(Cu2+)=1 mol/L时,CuCl(s)稳定区域的电极电位是0.137~0.537 V,pH为0~5.65。由此可见,若要生成CuCl沉淀必须严格控制反应条件。这些条件包括电位、pH、总铜浓度以及温度等。这些条件的变化范围有限,幅度小,因此,必须找到能显示与电位、pH、总铜浓度有关的指示电极,在线检测生成CuCl沉淀过程,以实现严格控制生成CuCl的沉淀条件[10]。
图2所示为Cu-Cl?-H2O体系的φ—lga(Cl?)图[11],由图可知,当体系中金属Cu、Cu2+和固态CuCl三相在α点上建立平衡时,达到除氯的最大限度,此时除氯效果最佳。
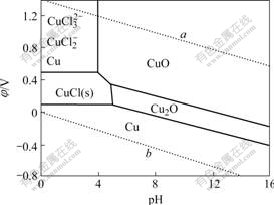
图1 Cu-Cl--H2O体系的φ—pH图
Fig.1 Potential—pH diagram for Cu-Cl?-H2O system
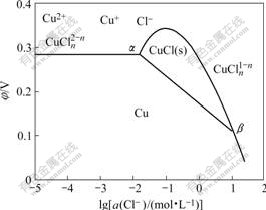
图2 Cu-Cl?-H2O体系的φ—lga(Cl?)图
Fig.2 Potential—lga(Cl?) diagram for Cu-Cl?-H2O system
体系中金属Cu、Cu2+和固态CuCl三相平衡之间的相互转化反应为

以上三相平衡建立时,φ(Cu2+/Cu)=φ[Cu2+/CuCl (s)],而且φ[Cu2+/CuCl(s)]决定于溶液中Cu2+与Cu+、Cl?活度之比,故可用惰性金属电极测得。因此,在向溶液中加入锌粉的过程中,只要测定铜电极和铂电极在溶液中的电极电位。当这两个电位首次相等时即表示体系在α点外建立了三相平衡状态[11]。根据φ—lga(Cl?)图可知,这时除氯效果达到最佳,可停止加锌粉。
2 实验
2.1 实验原料
实验原料主要如下:
五水硫酸铜(CuSO4?5H2O),分析纯;锌粉,分析纯,纯度为90%;
模拟工业ZnSO4浸出液的配制过程为:用去离子水溶解ZnSO4(分析纯)和NH4Cl(分析纯)配制成Zn2+含量120 g/L,Cl?含量2.7 g/L的溶液,并用硫酸调节溶液pH= 1.0。
实际工业ZnSO4浸出液为某锌厂的ZnSO4浸出液,溶液主要成分为Zn2+含量120g/L,Cl?含量2.2 g/L,pH约为1.0。
2.2 实验装置图
实验装置图如图3所示,其中用于测电位差的仪器为直流数字毫伏计,型号PZ158/1,分辨率为1 μV,其它为常用仪器设备。
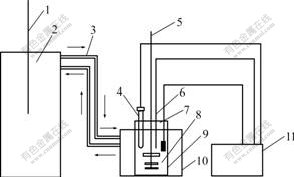
图3 实验装置示意图
Fig.3 Schematic diagram of experiment device: 1— Thermometer; 2—Stainless water thermostat; 3— Connecting hoses; 4—Saturated calomel reference electrode with double salt bridge; 5—Electric agitator; 6—Copper wire electrode; 7—Platinum electrode; 8—ZnSO4 solution; 9—Beaker; 10— Water bath sheath; 11—Millivoltmeter
2.3 实验步骤
2.3.1 加入锌粉除氯
取1 L ZnSO4浸出液置于2 L的烧杯中,加入50.440 g CuSO4?5H2O并溶解,体系中Cu2+含量0.2 mol/L。按照图3所示连接好实验设备,溶液温度为333.15 K时开始向溶液中逐量分批加锌粉,每次加入0.30 g左右。当电极指示的读数稳定后记下两个指示电极对应的读数,并停止搅拌。待溶液分层较明显时移取上层溶液约20 mL进行过滤,用于随后的Cl?含量分析。然后继续开始搅拌,进行下一次向溶液中加入锌粉的操作;直至铜丝电极和铂片电极指示的电极电势达到相同值时,停止加入锌粉和相继的操作,并结束实验。
2.3.2 Cl?含量分析
1) 化学滴定法
采用硝酸银滴定:移取上层滤液10 mL,稀释至50.00 mL,以0.05 mol/L AgNO3标准溶液为滴定剂,加入10滴10%(质量体积分数)K2CrO3溶液作指示剂进行沉淀滴定分析,滴定至终点时溶液由浅黄色突变成桔红色。
当滤液中大量Cu2+存在时溶液呈深蓝色,严重干扰了滴定终点突变的敏锐程度[12]。所以在滤液中Cu2+浓度高的时候,不能采用化学滴定法分析。
2) Cl?选择电极法
这种方法检测Cl?含量范围很大,可检测Cl?含量范围为5~8 000 mg/L的溶液,并且不受溶液颜色的干扰。Cl?选择电极法分为标准曲线法和标准加入法[13]。
标准曲线法 由于本实验中溶液离子强度远大于NaCl标准溶液的离子强度,采用标准曲线法所得数据精确度不高,所以一般应采用标准加入法。
标准加入法 移取10 mL上述滤液至100 mL容量瓶中,定容并摇晃均匀,再转移至300 mL烧杯中。在该溶液中以双液接甘汞参比电极为负极,Cl?选择电极为正极,读取电位差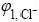 。然后加入1 mL浓度为cs的KCl标准溶液(cs约为欲测试液中Cl?浓度cx的100倍),混合均匀后读取选择电极在该溶液中的电位差
。然后加入1 mL浓度为cs的KCl标准溶液(cs约为欲测试液中Cl?浓度cx的100倍),混合均匀后读取选择电极在该溶液中的电位差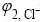 ,得到两次读数之差Δφ。由标准加入法公式[14]可知,滤液中Cl?的浓度为:
,得到两次读数之差Δφ。由标准加入法公式[14]可知,滤液中Cl?的浓度为:

3 结果与讨论
3.1 ZnSO4模拟浸出液加锌粉脱氯
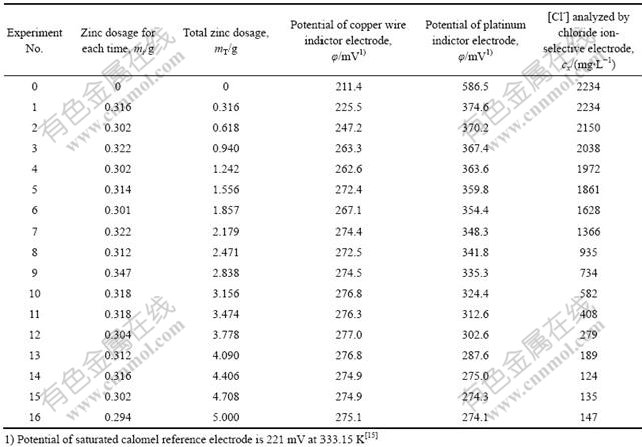
在水浴温度为333.15 K时,向1 L ZnSO4模拟浸出液中添加锌粉除氯,实验数据如表1所示。
由表1中实验数据可知,当第15次添加锌粉时,铜丝电极和铂片电极指示的电极电势值开始相交,此时φ(Cu2+/Cu)与φ[Cu2+/CuCl(s)]最接近,溶液体系中金属Cu、Cu2+和固态CuCl在α点处建立三相平衡,浸出液中Cl?含量达到最小。此后继续加入锌粉,Cl?含量上升,CuCl(s)开始返溶。实验数据与从Cu-Cl?-H2O多相体系的φ—lga(Cl?)图分析的结论完全吻合,这说明在氯化亚铜法除氯时可采用双电极指示来控制锌粉的加入量。
表1 水浴温度333.15 K时ZnSO4模拟浸出液除氯实验结果
Table 1 Experimental results of chloride removal from simulant ZnSO4 leachate at 333.15 K
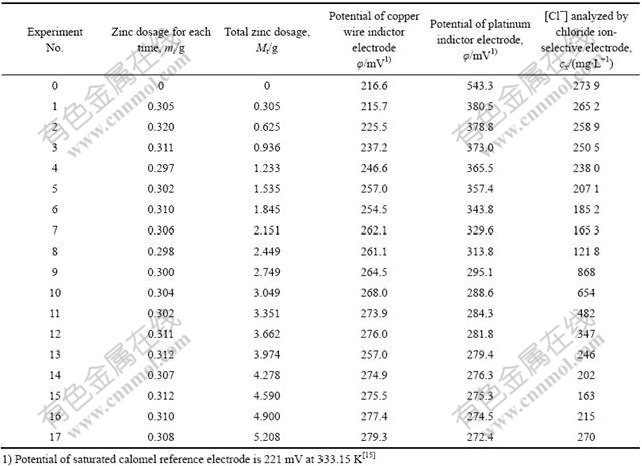
实验中锌粉的实际用量(4.590 g)约为理论所需量(2.522 g)的1.82倍,因为当锌粉加入到pH=1.0的浸出液后,不仅会与Cu2+反应,同时也可能与H+反应。这样就使得一部分锌粉消耗在还原氢离子反应中,而不能使锌粉全部用于将Cu2+还原成Cu+的反应,从而影响到脱氯效果。加入过量的锌粉弥补了还原氢离子所消耗的锌,使得Cu2+转化为Cu+的量有所增加,结果使脱氯率增大。
3.2 实际工业ZnSO4浸出液加锌粉脱氯
水浴温度为333.15 K时,在1 L实际工业ZnSO4浸出液中添加锌粉进行除氯实验,结果列于表2。由表2所列的实验数据可知,当第14次加入锌粉的时候,两个电极指示的电位最接近,可近似认为φ(Cu2+/Cu)=φ[Cu2+/CuCl(s)],溶液体系中金属Cu、Cu2+和固态CuCl在α点上建立三相平衡,浸出液中Cl?含量达到最小。继续加入锌粉,氯离子含量上升,CuCl(s)发生返溶。
表2 在水浴温度333.15 K时实际工业ZnSO4溶液除氯实验结果
Table 2 Experimental results chloride removal from industrial ZnSO4 leachate at 333.15 K
由实际工业ZnSO4浸出液除氯实验数据可知,采用铜丝和铂片双电极指示,同样可以控制锌粉的加入量使体系在α点上建立三相平衡,浸出液中Cl?含量达到最小,满足电解时Cl?含量<300 mg/L的要求。
3.3 ZnSO4浸出液中硫酸铜用量对脱氯效果的影响
氯化亚铜法除氯的反应是多相反应,其脱氯率的影响因素,主要有硫酸锌浸出液中Cu2+含量、锌粉添加量、反应时间和溶液酸度等[16?17]。其中最主要的影响因素是前两个,即Cu2+含量和锌粉添加量的影响。
要达到除氯后Cl?含量<300 mg/L,由金属铜、Cu2+和固态CuCl相互转化的反应方程式可得,理论加硫酸铜量应为0.077 3 mol/L。
在Cu2+含量分别为0.05、0.10、0.15、0.20和0.5 mol/L的硫酸锌浸出液中,分别加入锌粉除氯,当体系在α点上建立三相平衡时结束实验,测得各个溶液中残留Cu的含量,实验结果如图4所示。
由图4可以看出,当其他条件相同时,除氯效果随Cu2+含量的增加而提高;当Cu2+含量>0.15 mol/L时,开始达到除氯要求(Cl?含量<300 mg/L);当Cu2+含量高于0.2 mol/L时,继续升高Cu2+含量,除氯效果没有很明显的提高。
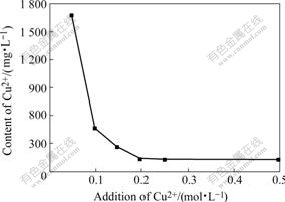
图4 硫酸铜的加入量对脱氯效果的影响
Fig.4 Effect of CuSO4 dosage on chloride removal effect
除氯反应方程式为:Cu2++Cu+2Cl?→2CuCl(s),由化学热力学和动力学原理可知,Cu2+含量越大,该反应向正方向进行得越彻底,除氯效果就越好,同时反应速度也越快,实验结果与其吻合。
综上可知,要达到除氯后Cl?含量<300 mg/L,实际加入硫酸铜量至少为0.15 mol/L,约为理论所需量的2倍。未用于沉淀氯反应的Cu2+含量被过量的锌粉还原为金属铜而进入沉淀,不会给硫酸锌浸出液带入杂质。
4 结论
1) 不论模拟ZnSO4浸出液脱氯,还是实际工业ZnSO4浸出液脱氯,实验证实都可以用双电极指示控制锌粉的添加量。在加锌粉过程中,用铜丝电极和铂片电极为指示电极监测体系的电位变化,当两种电极指示的电位达到相同值,即φ(Cu2+/Cu)= φ[Cu2+/CuCl (s)]时,溶液中的氯离子浓度达到最低值,除氯效果最好。
2) 氯化亚铜法除氯效果随Cu2+含量的增加而提高;要达到除氯要求,实际加入硫酸铜量至少为0.15 mol/L,约为理论所需量的2倍;当Cu2+含量高于0.2 mol/L时,除氯效果没有很明显的提高。
REFERENCES
[1] 彭容秋. 重金属冶金学[M]. 长沙: 中南大学出版社, 2004.
PENG Rong-qiu. Metallurgy of heavy metals[M]. Changsha: Central South University Press, 2004.
[2] Chen Y Y, Chung S C and Shih H C. Studies on the initial stages of zinc atmospheric corrosion in the presence of chloride[J]. Corrosion Science, 2006, 48(11): 3547?3564.
[3] 朱晓军, 朱建华. 脱氯技术现状与研究进展[J]. 化工生产与技术, 2005, 12(1): 24?28.
ZHU Xiao-jun, ZHU Jian-hua. Current situation and research progress of dechlorination technology[J]. Chemical Production and Technology, 2005, 12(1): 24?28.
[4] 陈家镛. 湿法冶金的研究与发展[M]. 北京: 冶金工业出版社, 1998.
CHEN Jia-yong. Research and development of hydrometallurgy[M]. Bejing: Metallurgica Industry Press, 1998.
[5] 许克昌, 杨建兴, 吴 慧. 锌熔铸浮渣中氯的脱除[J]. 有色金属(冶炼部分), 2004(5): 12?13.
XU Ke-chang, YANG Jian-xing, WU Hui. De-chlorine from floating slag of zinc casting[J]. Nonferrous Metals (Extractive Metallurgy), 2004(5): 12?13.
[6] 李 春, 李自强, 张 颖, 易 音. 活性铜粉从湿法炼锌浸液中脱氯[J]. 有色金属, 2002(1): 30?36.
LI Chun, LI Zi-qiang, ZHANG Ying, YI Yin. Chloride removing from zinc neutral leachate with nascent copper by cuprous chloride recipitation[J]. Nonferrous Metals, 2002(1): 30?36.
[7] 李 岚, 徐家振, 贺家齐, 郎晓珍, 符 岩, 白汝孝, 徐忠福, 黄敬庭. 氧化锌烟尘脱氯研究[J]. 中国有色金属学报, 1998, 8(2): 409?411.
LI Lan, XU Jia-zhen, HE Jia-qi, LANG Xiao-zhen, FU Yan, BAI Ru-xiao, XU Zhong-fu, HUANG Jing-ting. Study on chloride removal from zinc oxide dust[J]. The Chinese Journal of Nonferrous Metal, 1998, 8(2): 409?411.
[8] Zabaleta J, Carlos M. New developments for removal of chloride and potassium from the recovery cycle[J]. New Applied Technology Conference, 2004: 423?430.
[9] 傅崇说, 郑蒂基. 关于Cu-Cl?-H2O系的热力学分析及电位—pH图[J]. 中南矿冶学院学报, 1980(3): 12?24.
FU Chong-yue, ZHENG Di- ji. Thermodynamic analysis on the Cu-Cl?-H2O system and its potential—pH diagrams[J]. J Cent South Inst of Mining and Metall, 1980(3): 12?24.
[10] 孙 驰, 余仲兴, 周邦娜, 陈世琯. 铜氨水系中一价铜氨络离子的氧化[J]. 中国有色金属学报, 1999, 9(4): 821?826.
SUN Chi, YU Zhong-xing, ZHOU Bang-na, CHEN Shi-guan. Oxidation of ammoniacal cuprous complex ion in copper-ammonia-water system[J]. The Chinese Journal of Nonferrous Metal, 1999, 9(4): 821?826.
[11] 李皓月, 郑蒂基, 傅崇说. 关于Cu-Cl?-H2O系的平衡研究[J]. 中南矿冶学院学报, 1982(3): 39?45.
LI Hao-yue, ZHENG Di-ji, FU Chong-yue. Study on reaction equilibriums of Cu-Cl?-H2O system[J]. J Cent South Inst of Mining and Metall, 1982(3): 39?45.
[12] Wardak C, Marczewska B, Lenik J. Influence of ionic and nonionic surfactants on analytical parameters of ion-selective electrodes based on chelating active substances[J]. Electrochemical Acta, 2006, 51(11): 2267?2272.
[13] 贾梦秋, 杨文胜. 应用电化学[M]. 北京: 高等教育出版社, 2004: 215?218.
JIA Meng-qiu, YANG Wen-sheng. Applied electrochemistry[M]. Beijing: Higher Education Press, 2004: 215?218.
[14] Mahajan R K, Kaur R, Tabassum S. Cu(Ⅱ) complexes as receptor molecules for development of new chloridesensors[J]. Electrochemical Acta, 2006, 52(2): 408?414.
[15] Lee S U, Ahn J C, Kim D H, Hong S C, Lee K S. Influence of chloride and bromide anions on localized corrosion of 15% Cr ferritic stainless steel[J]. Mater Sci Eng A, 2006, 434(25): 155?159.
[16] 屠海令, 赵国权, 郭青蔚. 有色金属冶金、材料、再生与环保[M]. 北京: 化学工业出版社, 2002: 487?492.
TU Hai-ling, ZHAO Guo-quan, GUO Qing-wei. Metallurgy, material, regeneration and environmental conservation of nonferrous metals[M]. Beijing: Chemical Industry Press, 2002: 487?492.
[17] LI Zuo-peng, DU Zheng-yin, GU Yan-long, ZHU Lai-ying, ZHANG Xiao-ping, DENG You-quan. Environmentally friendly and effective removal of Br? and Cl? impurities in hydrophilic ionic liquids by electrolysis and reaction[J]. Electrochemistry Communications, 2006, 8(8): 1270?1274.
收稿日期:2006-10-10;修订日期:2007-02-20
通讯作者:李元高,研究员;电话:13975120950; E-mail: liyuangao202@sina.com.cn
(编辑 龙怀中)