Al2Ca金属间化合物对AZ31镁合金晶粒细化的影响
来源期刊:中国有色金属学报(英文版)2016年第5期
论文作者:姜中涛 蒋斌 章建跃 戴甲洪 杨青山 杨琴 潘复生
文章页码:1284 - 1293
关键词:AZ31镁合金;Al2Ca;晶粒细化;机理
Key words:AZ31 magnesium alloy; Al2Ca; grain refinement; mechanism
摘 要:采用真空熔炼方法制备Al2Ca金属间化合物并将其添加到AZ31镁合金中,研究其添加量对铸态AZ31镁合金晶粒细化的影响,同时讨论其晶粒细化机理。结果表明:添加1.1% Al2Ca(质量分数)可使得铸态AZ31镁合金晶粒尺寸从354 μm细化到198 μm,且经Al2Ca细化后,合金晶粒的热稳定性良好。晶粒细化的机理是溶质效应和Al2Ca的异质形核协同作用。
Abstract: The Al2Ca intermetallic compound was prepared by melting process in a vacuum induction furnace. And the Al2Ca compound was added in as-cast AZ31 alloys for grain refinement. The effect of its additional levels on grain refinement of as-cast AZ31 alloy was investigated and the mechanism of the grain refinement was discussed. The results reveal that the addition of 1.1% Al2Ca (mass fraction) decreases the average grain size of as-cast AZ31 alloy from 354 to 198 μm. And the thermal stability of the grains refined by Al2Ca is superior. The grain refining mechanism is attributed to the combined effects of solute and heterogeneous nucleation from the Al2Ca.
Trans. Nonferrous Met. Soc. China 26(2016) 1284-1293
Zhong-tao JIANG1,2, Bin JIANG1,3, Jian-yue ZHANG1, Jia-hong DAI1,
Qing-shan YANG3, Qin YANG4, Fu-sheng PAN1,3
1. National Engineering Research Center for Magnesium Alloys, College of Materials Science and Engineering,
Chongqing University, Chongqing 400044, China;
2. Research Institute for New Materials Technology, Chongqing University of Arts and Sciences,
Chongqing 402160, China;
3. Chongqing Academy of Science and Technology, Chongqing 401123, China;
4. Changan Auto Global Research and Development Center, Chongqing 401120, China
Received 6 June 2015; accepted 3 March 2016
Abstract: The Al2Ca intermetallic compound was prepared by melting process in a vacuum induction furnace. And the Al2Ca compound was added in as-cast AZ31 alloys for grain refinement. The effect of its additional levels on grain refinement of as-cast AZ31 alloy was investigated and the mechanism of the grain refinement was discussed. The results reveal that the addition of 1.1% Al2Ca (mass fraction) decreases the average grain size of as-cast AZ31 alloy from 354 to 198 μm. And the thermal stability of the grains refined by Al2Ca is superior. The grain refining mechanism is attributed to the combined effects of solute and heterogeneous nucleation from the Al2Ca.
Key words: AZ31 magnesium alloy; Al2Ca; grain refinement; mechanism
1 Introduction
Recently, magnesium alloys have been widely concerned in applications for the automobile and aerospace industries as a structure material due to their low specific strength and energy consumption [1,2]. However, for the most commercially and commonly used magnesium alloys, such as AZ31, AZ61 and AZ91, their mechanical properties and performance are relatively low and cannot meet the requirement of many applications [1]. It is well known that the grain size is a very important factor for overcoming this shortcoming of these alloys, and according to Hall-Petch equation, high strength can be attained with fine-grained magnesium alloys [3,4].
In Al-free Mg alloys, Zr is an extremely effective nucleation agent for the solidification of Mg alloys. Unfortunately, in commercially used Al-bearing Mg alloys, no suitable or effective nucleants have yet been found. Nonetheless, several approaches have been developed. These approaches mainly include superheating [5], carbon inoculation [6], adding suitable grain refiners and alloying elements, such as Ca, Sr [7], RE [8,9], Ti-B [10], Mn [11], ZnO [12], AlN [13] and Al2Y [14]. Due to simplicity and good adaptability to alloy’s compositions, the addition of small amount of alloying elements or grain refiner has become more popular in industrial application.
Ca is one of the most important alloying elements for Mg alloys due to low cost and lightness compared with other elements. In recent years, Ca has been used to improve the creep and high temperature performances of the Mg alloys. Mg-Al-Ca alloys revealed excellent creep resistance and elevated temperature properties due to the formation of high melting point Ca-containing phases with various sizes located both at the grain boundaries and within grains interior [15]. The addition of Ca to the AZ31 and AZ91 alloys can increase the high temperature properties because of the existence of (Al,Mg)2Ca phases [16,17]. HOMMA et al [18] reported the precipitation of Al2Ca plates during the creep test of Mg-Al-Ca-Mn alloys which had better creep properties. Additionally, some investigations reported that the addition of Ca into Mg-Al alloys will produce an effective grain refinement. Obvious microstructure refinement in AZ31 [19], Mg-5%Al [20], AM60 [21] and AZ91D alloys [22] was observed with Ca additions. Some investigators thought about the grain refinement mechanism of addition of Ca into Mg-Al alloys as the higher growth restriction factor (GRF) [23] of Ca in Mg alloy, some thought about it as the heterogeneous nucleation of in situ formed Al2Ca compounds in Mg alloys [19]. However, the refinement mechanism of Ca in Mg alloys is not fully understood and unfortunately, and the addition of Ca in AZ31 melt tends to increase the viscosity of the melt [13] and leads to the surface defect [24].
Therefore, in this work, in order to study the effect of Al2Ca compound addition on the grain refinement of Mg-Al alloys, Al2Ca intermetallic compound was prepared by melting process in a vacuum induction furnace under an argon atmosphere. Then, it was added into AZ31 Mg alloy melt to check the grain refining effects on as-cast AZ31 magnesium alloy and the possible refining mechanism.
2 Experimental
The Al2Ca intermetallic compound was synthesized by pure Al (>99.9%) and pure Ca (>99.9%) through melting reaction process at 1023 K using an alumina crucible in a vacuum induction furnace under an argon atmosphere. The Al/Ca molar ratio was 2:1. After the melting reaction, the products were held for about 10 min, then cooled with furnace and the primary Al2Ca block was achieved. The agate balls with a diameter of 10 mm were used as the milling media and mixed with the starting material at a ball-to-powder mass ratio of 10:1. The Al2Ca block and milling balls were put into the agate jar, and then mixed at a speed of 50 r/min for 12 h. Finally, the Al2Ca powder was obtained.
A commercial AZ31 Mg alloy was melted in a mild steel crucible in an electric resistance furnace under the protection of CO2+0.5% SF6 (volume fraction) mixture gas. Then, 0.5% Al2Ca, 1.1% Al2Ca, 1.7% Al2Ca and 3.5% Al2Ca powders were added into the AZ31 Mg alloy melt at about 993 K, respectively. The melt was stirred and held for 10 min and then poured into a steel mold (d20 mm × 90 mm) preheated to 473 K in order to obtain a sample ingot. The actual chemical compositions of the experimental alloys were inspected by an XRF 800CCDE X-ray fluorescence spectrometer and results are listed in Table 1. For grain morphology observation, all as-cast samples were cut in the horizontal direction at the location of 15 mm from the bottom of the ingots. Moreover, in order to clearly show the grain boundaries of the samples and estimate the thermal stability of the fine grains, the solid solution treatment of the samples was carried out at 688 K for 10 h and followed by water quenching.
Table 1 Chemical compositions of alloys studied (mass fraction, %)

In order to identify the precipitation sequence of the Al2Ca phase and the α-Mg phase, a thermal analysis method was used to detect whether the Al2Ca precipitates during solidification. Before cooling, a thermocouple was placed at the center of the mild steel crucible with its tip set at 10 mm from the bottom of the crucible. The alloy melt was cooled in the furnace. The cooling curves were recorded by a datalogger and computer. In addition, to study the stability of Al2Ca in liquid Mg, the Al2Ca/Mg solid-liquid diffusion couple was prepared by traditional method. Pure Mg and Al2Ca compound block (melting point about 1352 K) were used. A rectangular piece of Al2Ca compound block was polished with 800-grit SiC paper, and then cleaned with acetone to make sure an oxide-free surface. Pure Mg was melted under a protective atmosphere of CO2+0.5%SF6. Al2Ca compound block was immediately submerged into the Mg melt, and isothermally held at 993 K for 10 min, and then the samples were quenched in cold water.
These samples were ground, polished and then etched with a solution of 2.5 g picric acid + 45 mL ethanol + 2.5 mL acetic acid + 5 mL distilled water and then the grain size of each sample was examined using polarized light in Olympus optical microscope. The obtained color images were analyzed by Image-Pro Plus 5.0 software. The phase identification was analyzed by a Dimax 2500PC type X-ray diffractometer (XRD) operated at 40 kV and 30 mA. The microstructure was observed by a scanning electron microscope (SEM) with energy dispersive spectrometer (EDS).
3 Results and discussion
3.1 Characteristics of Al2Ca intermetallic compound
Figure 1 presents the XRD pattern of the Al-Ca intermetallic compound. It can be seen that the main compound in the product is Al2Ca and small amount of Al4Ca phase. In Al-Ca binary phase diagram [25], there have been two compounds, i.e., Al2Ca and Al4Ca. When the Al/Ca molar ratio is 2:1, the product is Al2Ca. Due to the little burning of Ca under the limited vacuum degree, the Al4Ca compound is present in the products. Figure 2(a) shows the image of the Al2Ca block sample. As the compound is very brittle, it is easy to crush and ball-mill into powder, as shown in Fig. 2(b). It can be seen that the powder is composed of anomalous particles with sizes of 5-10 μm.

Fig. 1 XRD pattern of Al2Ca intermetallic compound
3.2 Grain refining performance of Al2Ca intermetallic compound
In order to identify the phase composition of the alloys, the as-cast alloys were examined by XRD and the patterns are shown in Fig. 3. The AZ31 alloy consists only of α-Mg phase. Extra peak was reproducibly identified to be the Al2Ca phase in the spectra in the AZX3110, AZX3115 and AZX3130 alloys, respectively. The absence of the Al2Ca phase in the AZX3105 alloy is presumably due to the relatively small addition amount of the Al2Ca compound. Figure 4 shows the typical as-cast microstructures of the AZ31 alloys and Fig. 5 illustrates the variation of the average grain size with different addition levels of Al2Ca. Without any Al2Ca addition, the average grain size of AZ31 alloy is about 354 μm (Fig. 4(a)). With the increase of addition level of Al2Ca powder, the average grain size is remarkably reduced. When the addition of the Al2Ca is 1.1% (mass fraction), the average grain size decreases to 198 μm (Fig. 4(c)), i.e., the smallest grain size. However, with the further increase of Al2Ca addition level from 1.1% to 3.5% (mass fraction), the average grain size has a trend to increase (Figs. 4(d) and (e)). Therefore, it is clear that the Al2Ca compound can efficiently refine the as-cast AZ31 Mg alloy.

Fig. 2 SEM images of Al2Ca intermetallic compound obtained by casting
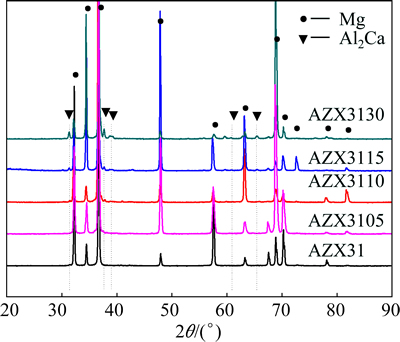
Fig. 3 XRD patterns of as-cast alloys
In order to clearly reveal the grain boundaries and distribution of the second phases, the as-cast samples of the experimental alloys were subjected to a solution heat treatment. The optical micrographs of the alloys after solution heat treatment are shown in Fig. 6. And the corresponding variation of the average grain sizes with different additions of Al2Ca is shown in Fig. 7. It is obvious that the change trend of grain size of solution- treated alloys with the increase of Al2Ca is the same as that of the as-cast alloys, similar to Fig. 5. However, after solution treatment at 688 K for 10 h, the average grain size without addition of Al2Ca alloy increases to 400 μm, as shown in Fig. 6(a). When Al2Ca contents are 1.1% and 1.7%, the average grain sizes are 200 and 223 μm, respectively, which is almost the same as that of the as-cast one. This indicates that the refined grains have high thermal stability. Next, it is worth noting that the grain boundaries of AZ31 alloy with addition of 3.5% Al2Ca are not be revealed, due to too many second phases distributed at the grain boundary. In order to observe the grain size, polarization images should be used again, as shown in Fig. 6(e), meanwhile, optical micrograph contained a grain (marked in red) in Fig. 6(e) is also overlapped.

Fig. 4 Optical micrographs of as-cast AZ31 alloys with different addition levels (mass fraction) of Al2Ca
Fig. 5 Grain size variation of as-cast AZ31 magnesium alloys with addition level of Al2Ca
Figure 8 presents the microstructures of the solution-treated AZ31 Mg alloy with different levels of Al2Ca addition. LIU et al [26] reported that the dissolution of Al2Ca into the Mg matrix is difficult during the solution treatment of alloys at 688 K. It is found that the alloying phases exist in the matrix of heat-treated alloys, shown as the bright parts in Fig. 8. The granular and irregular mass alloying phases may be Al-Mn intermetallic compound in the heat-treated AZ31 alloy without addition of Al2Ca compound, as shown in Fig. 8(a). In general, the Mg17Al12 and Al-Mn phases may exist in the as-cast AZ31 alloy. But, after solution treatment at 688 K for 10 h, Mg17Al12 phase is dissolved in the matrix. Besides, after the addition of the Al2Ca powders, the volume fraction of the bright parts rises with the increase of addition content of Al2Ca. For AZ31 alloy with 1.1% Al2Ca powder, the irregular shaped alloying phases are distributed not only inside the grains, but also along the grain boundary, as shown in Fig. 8(c). The distribution and morphology of alloying phases are almost no changed, but the size of the second phase is larger, compared with Figs. 8(c) and (d). As the Al2Ca content increases to 3.5%, semi-continuous alloying phases are so thick that it is difficult to identify grain boundaries of alloys, as shown in Fig. 8(e).

Fig. 6 Optical micrographs of AZ31 alloys after solution heat treatment with different amounts of Al2Ca
Fig. 7 Grain size variation of AZ31 alloys after solution heat treatment with different addition levels of Al2Ca
Meanwhile, it also reveals that the semi-continuous alloying phase forms probably during the eutectic solidification process and has a lamella-type morphology (the enlarged image in the upper right corner of Fig. 8(e)).
3.3 Grain refinement mechanism
Figure 9 shows SEM image and EDS analysis of AZ31 alloy with the addition of 1.1% Al2Ca powder. The chain-like and granular shape Al2Ca compound is distributed along the grain boundary and inside grains, respectively, such as points A and B. In addition, there also exists irregular massive Al-Mn compound within grains and Al-Mn-Ca compound near the grain boundary. In this work, the notable grain refinement of the AZ31 alloy is obtained by adding 1.1% Al2Ca compound (Fig. 4(c)). Although a small number of Al-Mn-Ca compounds (like point C in Fig. 9) exist in the alloy, the Mn content is the consistent as well as its contribution to grain refinement. So, the Al2Ca compound in the melt and solidification process plays a decisive role in the grain refinement of the AZ31 alloy.

Fig. 8 Microstructures of heat-treated AZ31 alloys with different additions of Al2Ca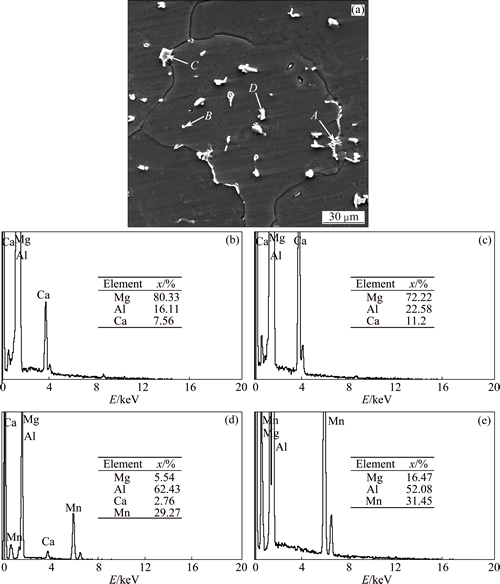
Fig. 9 SEM image (a) and EDS analysis of points A (b), B (c), C (d) and D (e) of AZ31 alloy with addition of 1.1% Al2Ca
In general, the grain refinement of polycrystalline materials is mainly determined by enhancing the nucleation rate in the melt and/or reducing the growth of grains [27,28]. So, the main approaches of grain refinement are adding potent nucleating agent, increasing undercooling and holding back grain boundary sliding. In this work, Al2Ca compound is adopted directly rather than Ca. So, the grain refinement of the AZ31 alloy obtained by adding Al2Ca seems to be the heterogeneous nucleation of primary α-Mg on the Al2Ca particles. In fact, some Al2Ca particles are often found in the central region of α-Mg grains (as shown in Figs. 8 and 9). In addition, the early study [19] found that the grain refinement through addition of Ca in the AZ31 alloy is attributed to the inoculation effect of Al2Ca particles formed in the melts, and crystallographic examination of relationship between Al2Ca and Mg using the edge-to-edge matching model indicates that Al2Ca particles are effective inoculants for heterogeneous nucleation of Mg. However, the solid particles for heterogeneous nucleation not only need to have a good lattice matching, but also need to be good stability in the liquid phase.
From Figs. 8 and 9, it is obvious that the morphology of the Al2Ca phase in the alloys is extremely different from pre-added particles of Al2Ca (as shown in Fig. 2(b)). It can presume that the Al2Ca compound can dissolve into AZ31 alloy melt, and subsequently precipitate during solidification, although the melting point of Al2Ca (1079 °C) is higher than 720 °C of the melt temperature. ROKHLIN et al [29,30] reported that the Al2Ca phase soluble in solid Mg is in equilibrium with the Mg solid solution in the Mg-Al-Ca system.
In order to validate the idea, the Mg/Al2Ca liquid-solid diffusion couple was prepared in Mg melt at 720 °C. Line scanning can characterize the diffusion of elements near the interface. The result of line scan (Fig. 10) obviously shows the mutual diffusion for Mg, Al and Ca elements. It can also show the possibility of the formation of intermetallic compounds. The compositions of these compounds were analyzed by the EDS. The compositions of the lamella-type phase (labeled as A) were found to be 64.6% Mg, 25.2% Al and 11.2% Ca (mole fraction). The compositions of the irregular bulk phase (labeled as B) was found to be 16.6% Mg, 56.1% Al and 27.3% Ca (mole fraction). And the composition of another lamella-type phase (labeled as C) was found to be 81.9% Mg, 12.3% Al and 5.8% Ca (mole fraction). This indicates that the mole ratios of Al to Ca in those compounds (labeled as A, B and C in Fig. 10) are all close to 2:1. Those compounds should be identified as Al2Ca. Besides, as shown in Fig. 10, there are two diffusion zones formed between Al2Ca and Mg alloys. Zone a represents the mixture of (Mg-Al2Ca) eutectic (labeled as A) with Al2Ca dendrites (labeled as B), and zone b represents the mixture of (Mg-Al2Ca) eutectic (labeled as C) with Mg dendrites. Only Mg and Al2Ca phases were found in this diffusion couple. This means that lamella-type eutectic Al2Ca was formed from the diffusion of Ca along with Al in Mg. Figure 11 shows the thermal analysis results for the investigated alloys, showing both the cooling curve and the variation of cooling rate with cooling time during solidification. The only one peak, can be clearly observed on the cooling rate curve (Fig. 11(a)), indicating the α-Mg phase nucleated at 627.8 °C in the AZ31 alloy, while the nucleation temperature of α-Mg phase is 623.7 °C in the AZX3110 alloy. Another peak at 516.8 °C (Fig. 11(b)) corresponds to Al2Ca phase precipitation in the AZX3110 alloy. That is close to precipitation temperature of the Al2Ca in the Mg-Al-Ca alloy [31].
Based on the above discussion and experimental data, we suggest that Al2Ca compound can dissolve into the Mg melt and subsequently re-precipitate during solidification under the condition of casting of the metal mould. So, at the initial stage of solidification, the solute segregation of Ca along with Al at solid/liquid interface can provide stronger constitutional undercooling for nucleation [32]. In addition, under the non-equilibrium solidification, it is possible that some Al2Ca precipitates firstly due to the limited solubility of Al2Ca [29]. This part of Al2Ca (point B in Fig. 9) was present in the undercooled layer and was activated as the potent nucleating agents of α-Mg. Therefore, this part of Al2Ca was observed in the center of the grains. The other Al2Ca was pushed to the grain boundaries and solidification end. As is known in Ref. [32], the grain refinement of Mg-Al by Ca addition can be improved further in the presence of potent nucleants.
It can be seen that grain refinement of AZ31 alloy by the Al2Ca compounds was mainly attributed to the combined effects of solute and heterogeneous nucleation. It can be seen from Fig. 5 that the grain size of AZ31 Mg alloy is minimum when the addition amount of Al2Ca is 1.1%. Combined with Fig. 8(c), Al2Ca particles are distributed not only inside the grains but also along the grain boundary with fine and dispersed granular. But excessive addition of Al2Ca powders is prone to aggregate to form clusters (Figs. 8(d) and (e)) and the nucleating particles are difficult to disperse. Correspondingly, the number of effective heterogeneous nuclei decreases, which decreases the grain refining efficiency of Al2Ca on AZ31 Mg alloy. A similar phenomenon has been reported in Ref. [33].

Fig. 10 Microstructures of liquid-solid Mg-Al2Ca diffusion couple at 720 °C with SEM-EDS line scanning
Fig. 11 Cooling curves and rate curves determined from thermal analysis
4 Conclusions
1) The Al2Ca intermetallic compound was prepared by pure metal melting process in a vacuum induction furnace under an argon atmosphere. This shows perfect grain refining effect for AZ31 alloy.
2) The Al2Ca compounds can be dissolved into the AZ31 alloy melt, subsequently re-precipitate during solidification under the non-equilibrium rapid solidification, and provide stronger constitutional undercooling for the grain refinement process.
References
[1] WU Guo-hua, YU Fan, GAO Hong-tao, ZHAI Chun-quan, YUAN Ping-zhu. The effect of Ca and rare earth elements on the microstructure, mechanical properties and corrosion behavior of AZ91D [J]. Materials Science and Engineering A, 2005, 408: 255-263.
[2] CHANG H W, QIU D, TAYLOR J A, EASTON M A, ZHANG M X. The role of Al2Y in grain refinement in Mg-Al-Y alloy system [J]. Journal of Magnesium and Alloys, 2013, 1: 115-121.
[3] WANG Jing-tao, YIN De-liang, LIU Jin-qiang, TAO Jun, SU Yan-ling, ZHAO Xiang. Effect of grain size on mechanical property of Mg-3Al-1Zn alloy [J]. Scripta Materialia, 2008, 59: 63-66.
[4] STJOHN D H, EASTON M A, QIAN M, TAYLOR J A. Grain refinement of magnesium alloys: A review of recent research, theoretical developments, and their application [J]. Metallurgical and Materials Transactions A, 2012, 44: 2935-2949.
[5] CAO P, MA Q, STJOHN D H. Mechanism for grain refinement of magnesium alloys by superheating [J]. Scripta Materialia, 2007, 56: 633-636.
[6] MOTEGI T. Grain-refining mechanisms of superheat-treatment of and carbon addition to Mg-Al-Zn alloys [J]. Materials Science and Engineering A, 2005, 413-414: 408-411.
[7] HIRAI K, SOMEKAWA H, TAKIGAWA Y, HIGASHI K. Effects of Ca and Sr addition on mechanical properties of a cast AZ91 magnesium alloy at room and elevated temperatures [J]. Materials Science and Engineering A, 2005, 403: 276-280.
[8] LI W P, ZHOU H, LI Z F. Effect of gadolinium on microstructure and rolling capability of AZ31 alloy [J]. Journal of Alloys and Compounds, 2009, 475: 227-232.
[9] TIAN X, WANG L M, WANG J L, LIU Y B, AN J, CAO Z Y. The microstructure and mechanical properties of Mg-3Al-3RE alloys [J]. Journal of Alloys and Compounds, 2008, 465: 412-416.
[10] WANG Ying-xin, ZENG Xiao-qin, DING Wen-jiang. Effect of Al-4Ti-5B master alloy on the grain refinement of AZ31 magnesium alloy [J]. Scripta Materialia, 2006, 54: 269-273.
[11] GU Xin-fu, ZHANG Wen-zheng, QIU Dong. A systematic investigation of the development of the orientation relationship in an fcc/bcc system [J]. Acta Materialia, 2011, 59: 4944-4956.
[12] FU H M, QIU D, ZHANG M X, WANG H, KELLY P M, TAYLOR J A. The development of a new grain refiner for magnesium alloys using the edge-to-edge model [J]. Journal of Alloys and Compounds, 2008, 456: 390-394.
[13] FU H M, ZHANG M X, QIU D, KELLY P M, TAYLOR J A. Grain refinement by AlN particles in Mg-Al based alloys [J]. Journal of Alloys and Compounds, 2009, 478: 809-812.
[14] QIU D, ZHANG M X, KELLY P M. Crystallography of heterogeneous nucleation of Mg grains on Al2Y nucleation particles in Mg-10wt.% Y alloy [J]. Scripta Materialia, 2009, 61: 312-315.
[15] LUO A L, BALOGH M P, POWELL B R. Creep and microstructure of magnesium-aluminum-calcium [J]. Metallurgical and Materials Transactions A, 2002, 33: 567-574.
[16] SUZUKI A, SADDOCK N D, JONES J W, POLLOCK T M. Solidification paths and eutectic intermetallic phases in Mg-Al-Ca ternary alloys [J]. Acta Materialia, 2005, 53: 2823-2834.
[17] LASER T, HARTIG C, NüRNBERG M R, LERZIG D, BORMANN R. The influence of calcium and cerium mischmetal on the microstructural evolution of Mg-3Al-1Zn during extrusion and resulting mechanical properties [J]. Acta Materialia, 2008, 56: 2791-2798.
[18] HOMMA T, NAKAWAKI S, KAMADO S. Improvement in creep property of a cast Mg-6Al-3Ca alloy by Mn addition [J]. Scripta Materialia, 2010, 63: 1173-1176.
[19] JIANG Bin, LIU Wen-jun, QIU Dong, ZHANG Ming-xing, PAN Fu-sheng. Grain refinement of Ca addition in a twin-roll-cast Mg-3Al-1Zn alloy [J]. Materials Chemistry and Physics, 2012, 133: 611-616.
[20] HAN L H, HU H, NORTHWOOD D O. Effect of Ca additions on microstructure and microhardness of an as-cast Mg-5.0wt.% Al alloy [J]. Materials Letters, 2008, 62: 381-384.
[21] KONDORI B, MAHMUDI R. Effect of Ca additions on the microstructure, thermal stability and mechanical properties of a cast AM60 magnesium alloy [J]. Materials Science and Engineering A, 2010, 527: 2014-2021.
[22] LI S S, TANG B, ZENG D B. Effects and mechanism of Ca on refinement of AZ91D alloy [J]. Journal of Alloys and Compounds, 2007, 437: 317-321.
[23] STJOHN D H, MA Q, EASTON M A, CAO P, HILDEBRAND Z. Grain refinement of magnesium alloys [J]. Metallurgical and Materials Transactions A, 2005, 36: 1669-1679.
[24] QIAN Bao-guang, GENG Hao-ran, TAO Zhen-dong, ZHAO Peng, TIAN Xian-fa. Effects of Ca addition on microstructure and properties of AZ63 magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(5): 987-991.
[25] ITKIN V P, ALCOCK C B, EKEREN P J V, OONK H A J. The Al-Ca (aluminum-calcium) system [J]. Bulletin of Alloy Phase Diagrams, 1988, 9: 652-657.
[26] LIU Man-ping, WANG Qu-dong, LIU Zi-li, YUAN Guang-yin, WU Guo-hua, ZHU Yan-ping, DING Wen-jiang. Behavior of Mg-Al-Ca alloy during solution heat treatment at 415 oC [J]. Journal of Materials Science Letters, 2002, 21: 1281-1283.
[27] LEE Y C, DAHLE A K, STJOHN D H. The role of solute in grain refinement of magnesium [J]. Metallurgical and Materials Transactions A, 2000, 31: 2895-2906.
[28] YAHIA A, QIU Dong, JIANG Bin, PAN Fu-sheng, ZHANG Ming-xing. Current research progress in grain refinement of cast magnesium alloys: A review article [J]. Journal of Alloys and Compounds, 2015, 619: 639-651.
[29] ROKHLIN L L, NIKITINA N I, VOLCHENKOVA V A. Magnesium-rich Mg-Al2Ca alloys [J]. Russian Metallurgy, 2006, 2: 185-188.
[30] ROKHLIN L L, DOBATKINA T V, NIKITINA N I, TARYTINA I E. Calcium-alloyed magnesium alloys [J]. Metal Science and Heat Treatment, 2009, 51: 164-169.
[31] JIANG Zhong-tao, JIANG Bin, ZHANG Jian-yue, XIA Xiang-sheng, PAN Fu-sheng. Microstructural evolution of Mg-4Al-2.5Ca alloy during solidification [J]. Materials Science Forum, 2015, 816: 486-491.
[32] NAGASICAMUNI B, RAVI K R. An analytical approach to elucidate the mechanism of grain refinement in calcium added Mg-Al alloys [J]. Journal of Alloys and Compounds, 2015, 622: 789-795.
[33] CHEN Ti-jun, WANG Rui-quan, HUANG Hai-jun, MA Ying, HAO Yuan. Grain refining technique of AM60B magnesium alloy by MgCO3 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1533-1539.
姜中涛1,2,蒋 斌1,3,章建跃1,戴甲洪1,杨青山3,杨 琴4,潘复生1,3
1. 重庆大学 材料科学与工程学院 国家镁合金材料工程技术研究中心,重庆 400044;
2. 重庆文理学院 新材料技术研究院,重庆 402160;
3. 重庆科学技术研究院,重庆 401123;
4. 长安汽车工程研究院,重庆 401120
摘 要:采用真空熔炼方法制备Al2Ca金属间化合物并将其添加到AZ31镁合金中,研究其添加量对铸态AZ31镁合金晶粒细化的影响,同时讨论其晶粒细化机理。结果表明:添加1.1% Al2Ca(质量分数)可使得铸态AZ31镁合金晶粒尺寸从354 μm细化到198 μm,且经Al2Ca细化后,合金晶粒的热稳定性良好。晶粒细化的机理是溶质效应和Al2Ca的异质形核协同作用。
关键词:AZ31镁合金;Al2Ca;晶粒细化;机理
(Edited by Wei-ping CHEN)
Foundation item: Projects (CSTC2013jcyjC60001, CSTC2013jcyjA50020, CSTC2014jcyjjq0041) supported by the Chongqing Science and Technology Commission, China; Projects (51531002, 51171212, 51474043) supported by the National Natural Science Foundation of China; Projects (2013DFA71070, 2013CB632200) supported by the National Science and Technology Program of China; Project (KJZH14101) supported by the Education Commission of Chongqing Municipality, China
Corresponding author: Bin JIANG; Tel: +86-13594190166; Fax: +86-23-65111140; E-mail: jiangbinrong@cqu.edu.cn
DOI: 10.1016/S1003-6326(16)64229-2

