用于去除水溶液中铜离子的环境友好的聚(对苯二胺)及其壳聚糖复合材料的制备和表征
来源期刊:中国有色金属学报(英文版)2015年第11期
论文作者:N. A. ABDELWAHAB E. A. AL-ASHKAR M. A. ABD EL-GHAFFAR
文章页码:3808 - 3819
关键词:铜去除;吸附;聚(对苯二胺)/壳聚糖复合材料;动力学;等温线
Key words:copper removal;adsorption;poly(p-phenylenediamine)/chitosan composite;kinetics;isotherms
摘 要:利用过硫酸铵作为氧化剂,在壳聚糖(Chi)中用对苯二胺(PPPDA)原位化学氧化聚合反应制备聚(对苯二胺)/壳聚糖复合材料。在添加铜前后,利用FT-IR光谱和SEM表征对苯二胺和对苯二胺/壳聚糖复合材料。利用振荡吸附法,采用1 g/L对苯二胺和对苯二胺/壳聚糖复合材料,在PPPDA和PPPDA/Chi复合材料的pH分别为5.0和6.0的条件下,吸附360 min,可以得到最大的铜去除量。PPPDA显示最大吸附量为650 mg/g,而复合材料的吸附量达到 573 mg/g。实验数值与Freundlich等温方程和伪二阶动力学模型吻合良好。含铜的对苯二胺及其复合材料可以被有效地再利用4个循环。相对于无吸附的状态,吸附获得的铜具有高的革兰氏阳性和革兰氏阴性菌抑菌效果。
Abstract: Poly(p-phenylenediamine)/chitosan (PPPDA/Chi) composite was prepared by in situ chemical oxidative polymerization of p-phenylenediamine (PPPDA) into chitosan (Chi) using ammonium persulphate (APS) as an oxidant. PPPDA and PPPDA/Chi composite were characterized by FT-IR spectra and SEM before and after copper loading. In batch adsorption method, the maximum removal of copper was experienced when 1 g/L of PPPDA and PPPDA/Chi composite dosages were used at pH 5.0 for PPPDA and 6.0 for PPPDA/Chi composite for 360 min for both sorbents. PPPDA showed adsorption capacity qemaxof 650 mg/g whereas its composite achieved qemaxof 573 mg/g. The experimental data correlate well with the Freundlich isotherm equation and the pseudo-second order kinetic model. The Cu(II), loaded PPPDA and its composite can be efficiently reused for as many as four cycles. The Cu(II)-loaded sorbents showed high antibacterial efficiency against Gram-positive and Gram-negative bacteria than their unloaded forms.
Trans. Nonferrous Met. Soc. China 25(2015) 3808-3819
N. A. ABDELWAHAB1, E. A. AL-ASHKAR2, M. A. ABD EL-GHAFFAR1
1. Polymers and Pigments Department, National Research Centre, 33 El Bohouth St. Dokki, Giza, P. O. 12622, Egypt;
2. Spectroscopy Department, Physics Division, National Research Centre, 33 El Bohouth St. Dokki, Giza, P. O. 12622, Egypt
Received 16 December 2014; accepted 6 April 2015
Abstract: Poly(p-phenylenediamine)/chitosan (PPPDA/Chi) composite was prepared by in situ chemical oxidative polymerization of p-phenylenediamine (PPPDA) into chitosan (Chi) using ammonium persulphate (APS) as an oxidant. PPPDA and PPPDA/Chi composite were characterized by FT-IR spectra and SEM before and after copper loading. In batch adsorption method, the maximum removal of copper was experienced when 1 g/L of PPPDA and PPPDA/Chi composite dosages were used at pH 5.0 for PPPDA and 6.0 for PPPDA/Chi composite for 360 min for both sorbents. PPPDA showed adsorption capacity qemax of 650 mg/g whereas its composite achieved qemax of 573 mg/g. The experimental data correlate well with the Freundlich isotherm equation and the pseudo-second order kinetic model. The Cu(II), loaded PPPDA and its composite can be efficiently reused for as many as four cycles. The Cu(II)-loaded sorbents showed high antibacterial efficiency against Gram-positive and Gram-negative bacteria than their unloaded forms.
Key words: copper removal; adsorption; poly(p-phenylenediamine)/chitosan composite; kinetics; isotherms
1 Introduction
High dosages of copper can cause health problems such as lesions in the central nervous system, Wilson’s disease, and gastrointestinal disturbance, moreover, it has toxic effect on aquatic organisms at low pH [1-8]. So, its removal from wastewater is important for public health protection. Oxidation [9,10], reduction [11], electro- chemical precipitation [12], ion exchange [13], and membrane ultrafiltration [14], are expensive, not eco-friendly and conventional. Besides, secondary pollutions may occur in effluents. In recent years, growing attention has been paid to adsorption for its high efficiency in sewages containing low heavy metal concentrations, relatively simple process and its reusability [15].
Among the adsorbents, chitosan as a high performance natural polymer is considered as outstanding candidate since it is nontoxic, reproducible, inexhaustible, biodegradable and environment-friendly [16,17]. Chitosan exhibits efficient adsorption properties due to its excellent chelating effects for containing abundant hydroxyl and amino groups on the chain backbone [18]. As reported in the literature, chitosan-coated sludge exhibited maximum adsorption capacity of 18.83 mg/g for Cu(II) ions [19]. In another research, it showed copper uptake of 1.8-2.2 mmol/g [20]. Furthermore, the maximum adsorption capacity of chitosan-coated PVC sorbent reached 87.9 mg/g for Cu(II) ions [21]. Also, sorption behaviour of chemically modified and acrylamide grafted chitosan for Cu(II) removal [22-24] has been studied. Moreover, the removal of Cu(II) by chitosan-g-poly(acrylic acid)/ attapulgite composite [25] and chitosan/zeolite composite [26] has been also investigated.
Intrinsic conducting polymers have attracted attention as ion exchangers, energy storage materials, corrosion-resistant coatings, catalysts and chemical sensors [27-29]. They also showed affinity to some metal ions due to their chelating properties and/or reduction properties [30-34]. In earlier studies, poly (1,8-diaminonaphthalene) and poly(4-sulphonic diphenylamine) were employed as novel sorbents for effective extraction of silver ions [32,33], while fine microparticles of poly(m-phenylenediamine) (PmPD) exhibited strong ability to adsorb lead ions from aqueous solutions [34]. In another study, PmPD exhibited Ag+ absorbability of 2073 mg/g [35], while in other works, the Ag+ adsorbance of PmPD can reach 1693 mg/g [36] and the highest Cr(VI) removal was 500 mg/g [37]. PmPD also achieved adsorption capacity of 108.5 mg/g for sulphate ion [38]. Electropolymerized poly (4-vinylpyridine) was also investigated for copper removal [39]. Moreover, the adsorption of Cd(II) from wastewater by polyaniline coated on sawdust was investigated [40]. Besides, Zn(II) removal from aqueous solutions using polypyrrole/ sawdust nanocomposite has been reported [41].
The antimicrobial activity of chitosan depends mainly on the formation of polymeric coating on the surface of cell membrane which prevents nutrients from entering the cell. Additionally, it can also diffuse into the cell and adsorb the electronegative substance in the cell and flocculate them. This causes disturbance in the normal physiological activities of the bacterial cell and results in cell death [42]. Polyanilines exhibited antibacterial activity due to their conductive doped structure that interacts with bacterial cell walls. Conducting polymers are reported to cause cell death by disrupting microbial cell walls by electrostatic contact [43].
Few researches have been found for the use of chitosan together with conducting polymers to remove heavy metals from wastewater. This includes the removal of Cr(VI) from aqueous solutions using poly (3-methyl thiophene) coated chitosan [44]. Recently, chitosan and polyaniline grafted chitosan showed the Cu(II) removal maximum adsorption capacity of 52.6 mg/g and 100 mg/g, respectively [45].
However, there is no study regarding the sorption of Cu(II) onto PPPDA and PPPDA/Chi composite. The main aim of this work is to study the efficiency of PPPDA and PPPDA/Chi composite for Cu(II) removal from aqueous solutions. The adsorption isotherms and kinetics in batch mode were investigated. Furthermore, various conditions like pH, contact time and sorbent dosage of metal ion were optimized for maximum sorption capacity. The reusability of PPPDA and PPPDA/Chi composite was studied.
2 Experimental
2.1 Materials
Chitosan (Chi) was provided by Aldrich with a deacetylation percentage of approximate 90% and average relative molecular mass 800000. p-phenylenediamine (PPDA), ammonium persulphate (APS) and acetic acid were obtained from Sigma. Copper sulphate pentahydrate (CuSO4·5H2O) (used to prepare the copper ion solutions in the adsorption experiments), sodium nitrate, hydrochloric acid and sodium hydroxide (used for pH adjustment) were supplied by Aldrich. All other chemicals used were of analytical grade and distilled water was used in the preparation of all solutions.
2.2 Preparation of PPPDA
As previously mentioned in Ref. [46], 0.01 mol/L APS in 50 mL 1 mol/L HCl was dropped into 50 mL of 1 mol/L HCl containing 0.01 mol PPDA with vigorous stirring under N2 atmosphere. The solution colour turns dark and the polymerization was carried out for 24 h at room temperature. After that, the precipitated polymer was filtered and washed with distilled water, methanol and finally with acetone and then dried at 60 °C for 24 h. The resulting polymer has cyclic structure comprising of 1,4-benzodiazine repeating units, the chelating site here is nitrogen atom of free amino group at the end of each chain or cyclic imino groups.
2.3 Preparation of PPPDA/Chi composite
The polymer composite was prepared as reported earlier [47,48]. In a typical procedure, 0.2 g chitosan was dissolved in 40 mL aqueous acetic acid (2%) for 24 h. 0.01 mol PPDA was dissolved in 50 mL 1 mol/L HCl and then added to Chi solution with stirring for 30 min to obtain homogeneous solution. 0.01 mol APS solution in 50 mL 1 mol/L HCl was dropped into PPDA/Chi mixture solution with continuous stirring under N2 atmosphere. The reaction was kept for 24 h at room temperature. After that, the precipitated polymer/ Chi composite was filtered, washed with distilled water, methanol and acetone. Finally, the polymer composite was dried at 50 °C for 24 h. PPPDA bonded to Chi by hydrogen bonding between OH and NH2 of Chi and NH of PPPDA. The chemical structure of PPPDA/Chi composite is presented in Scheme 1.
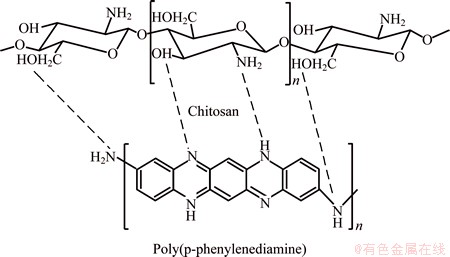
Scheme 1 Chemical structure of PPPDA/Chi composite
2.4 Characterization
The pH values of the solutions were determined by using an AD-11 pH meter (Adwa). The FT-IR spectra of PPPDA and PPPDA/Chi composite before and after copper adsorption were recorded in the frequency range of 4000-400 cm-1 using a Nexus 670 FT-IR spectro- photometer, Nicolet, USA. The morphologies of the PPPDA and PPPDA/Chi composite before and after adsorption were investigated through scanning electron microscopy (SEM, JEOL JXA-840A, Japan). Before SEM, the samples were coated with gold through anodic precipitation of gold ions onto sample surface under argon gas atmosphere and current 40 mA for 3-6 min using S150A SPUTTER Coater, Edwards, England. Thermal gravimetric analysis (TGA) was carried out using a Perkin Elmer thermogravimetric analyzer in the presence of N2 atmosphere from room temperature up to 600 °C with a heating rate of 10 °C/min.
2.5 Batch adsorption experiments
Adsorption experiments were performed, as reported earlier [47,48], by mixing 25 mg of polymer or polymer composite with 25 mL of copper ion solution of desired concentration in stoppered bottles. The solution pH was adjusted to the desired value with dilute HCl or NaOH solution using a pH meter with a resolution of ±0.01. The solution was agitated using magnetic stirrer with constant speed (300 r/min) for all experiments throughout the study at (30±0.5) °C. At the end of predetermined time intervals, the solution was filtered and the concentration of copper ions was measured by an Agilent Technologies GTA120 Graphite Tube Atomizer 200 Series AA atomic absorption spectrophotometer (AAS). The amount of copper ion adsorbed (Qe) was calculated according to the following equation:
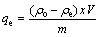
where qe is the amount of metal ions adsorbed by PPPDA or PPPDA/Chi composite, ρo and ρe are the metal ion concentrations in the solution initially and after adsorption, respectively, V is the volume of the solution and m is the mass of adsorbent used. Experimental variables considered were pH 2-6, sorbent dosage 1-4 g/L and agitation time 30-360 min.
2.6 Isotherm studies
Adsorption isothermal studies were carried out when 25 mL of different initial concentrations of Cu(II) ion solution (50-1000 mg/L) were mixed with 25 mg of adsorbents and stirred at 300 r/min for 360 min at 25 °C, the pH was 5.0 for PPPDA and 6.0 for PPPDA/Chi composite. The solution was filtered and the filtrate was analyzed for Cu(II) ion concentration using AAS. Freundlich, and Langmuir isotherms were adopted to quantify the sorption capacity of the studied sorbents for Cu(II) removal.
2.7 Kinetic studies
Adsorption kinetic studies were performed as follows: 1 g of PPPDA and PPPDA/Chi composite were added separately into 100 mL of Cu(II) ion solution with initial concentration of 500 mg/L and pH of 5.0 for PPPDA and 6.0 for PPPDA/Chi composite. The mixture was stirred magnetically at 25 °C and samples were taken from the solution at desired time intervals for the analysis of Cu(II) ion concentrations in the solution using AAS.
2.8 Reusability studies
4 g of each adsorbent was loaded with 50 mg/L Cu(II) solution at 25 °C. The mixture was stirred at 300 r/min for 360 min to reach equilibrium. The sorbent suspension was filtered and left to air dry. An accurate mass of 50 mg of each sorbent loaded with Cu(II) was mixed with 50 mL (0.01 mol/L) EDTA solution. The mixture was left for 24 h. After desorption, sorbent suspension was filtered and the final concentration of Cu(II) was analyzed by AAS. Desorption efficiency of each sorbent for Cu(II) is calculated as the percentage of the ratio between the amount of Cu(II) desorbed and amount of Cu(II) adsorbed.
2.9 Antibacterial properties
The antibacterial activity of PPPDA, PPPDA/Chi composite, optimized PPPDA-loaded Cu2+ and optimized PPPDA/Chi-loaded Cu2+ against Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa) cultures as Gram-negative bacteria strains and Staphylococcus aureus (S. aureus) and Bacillus subtilis (B. subtilis) cultures as Gram-positive bacteria strains were studied. In agar diffusion plate test, the bacteria reference strains were cultured on nutrient agar and incubated aerobically at 37 °C overnight. 100 μL of diluted cell suspension of bacteria was mixed with tested powder samples and added to Petri dish. The Petri dish was incubated at 37 °C for 24 h. After incubation, the growth of inhibition zone diameter was estimated.
3 Results and discussion
3.1 Characterization of prepared adsorbents
3.1.1 FT-IR spectra
Figure 1 depicts the FT-IR spectra of PPPDA and PPPDA/Chi composite before and after copper adsorption. The most important features for PPPDA (Fig. 1(a)) are following broad band at 3450 cm-1 attributed to N—H stretching, 2922 cm-1 is due to —CH stretching vibration, 1570 cm-1 attributed to NH— bending, 1335 cm-1 and 1165 cm-1 resulted from C—N stretching vibration. For the chemically synthesized PPPDA/Chi composite (Fig. 1(c)), it can be seen that the FT-IR spectra of the composite have the characteristic bands of both chitosan and PPPDA including —OH stretching of chitosan and —NH2 stretching of chitosan and PPPDA at 3444 cm-1, —NH2 and —NH bending of chitosan and PPPDA at 1680 cm-1, —CH bending of chitosan and PPPDA at 1390 cm-1 and C—O stretching of chitosan at 1019 cm-1. It was observed that there are some shifts in the peak frequencies upon composite formation and this could be attributed to hydrogen bonding between PPPDA and Chi. Furthermore, the intensity of the band characteristic to NH— stretching was reduced sharply. Some shifts in peaks frequencies were observed for PPPDA-loaded Cu2+ and PPPDA/Chi composite-loaded Cu2+, there are some shifts for the peaks corresponding to —OH and NH2 in Chi and —NH of PPPDA which may be taken as an indication for sorption between the two studied sorbents and copper (Figs. 1(b) and (d)).
3.1.2 Morphology
Figures 2(a)-(f) show SEM micrographs of PPPDA, PPPDA/Chi composite, PPPDA-loaded Cu2+ and PPPDA/Chi composite-loaded Cu2+, respectively. From these figures, it can be noticed that PPPDA (Fig. 2(a)) has irregular structure comprising adhered plates and agglomeration of granules together whereas in PPPDA/Chi composite (Fig. 2(b)) PPPDA plates and granules imbedded between chitosan thin flakes to give agglomeration and irregular morphology. The SEM micrographs of the PPPDA and PPPDA/Chi composite after copper sorption indicate significant changes in the sorbent morphology and the loading of copper was clearly observed in Figs. 2(c) and (d). Before copper sorption, the structure of PPPDA is porous and after copper sorption, these pores disappear and the structure seems cemented and closed firmly (Fig. 2(c)). Moreover, the PPPDA surface is completely coated with adsorbed copper and also between pores and this is an evidence for high adsorption capacity values for PPPDA. On the other hand, in the PPPDA/Chi composite-loaded Cu2+, the copper coated the granules and flakes surfaces (Fig. 2(d)).
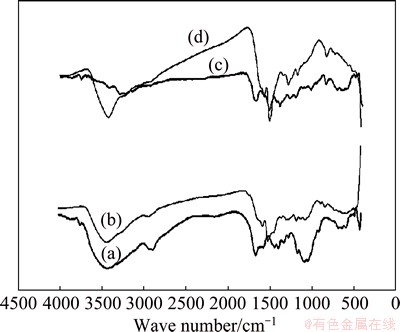
Fig. 1 FT-IR spectra of PPPDA (a), PPPDA-loaded Cu+2 (b), PPPDA/Chi (c) and PPPDA /Chi-loaded Cu+2 (d)
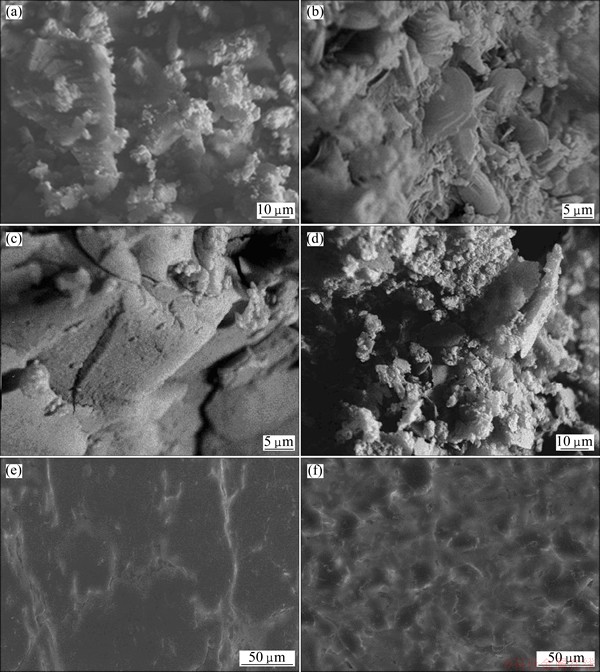
Fig. 2 SEM iamges of PPPDA (a), PPPDA /Chi composite (b), PPPDA-loaded Cu+2 (c) and its magnified form (d), PPPDA/Chi composite-loaded Cu+2 (e) and its magnified form (f)
3.1.3 TGA
The degradation and thermal stability behavior of PPPDA and PPPDA/Chi composite were evaluated by TGA under a nitrogen atmosphere. Figure 3 shows the TGA thermograms of PPPDA and PPPDA/Chi composite. When the temperature is raised up to 100 °C, a slight decrease in initial mass of PPPDA and PPPDA/Chi is due to loss of water molecules. Beyond 100 °C, a gradual mass loss was observed at 223 °C and this could be related to loss of dopant molecules and breaking of hydrogen bonds in the polymer composite. Finally, from 223 to 600 °C, a steep mass loss occurred and this is corresponding to decomposition of the polymers backbone. The polymer showed higher thermal stability than its composite.
3.2 Effect of pH
pH is considered as an important variable and greatly affects the solubility of the metal ions in the solution, and these metal ions replace some of the positive ions found in the active sites and also affect the degree of ionization of the adsorbate during the reaction [28]. Hence, the adsorption of metal ions by functional groups present on the PPPDA or PPPDA/Chi surface depends greatly on the pH. 25 mL samples having an initial concentration of 800 mg/L was transferred into a series of Erlenmeyer flasks, the pH of the samples was adjusted by 0.1 mol/L HCl and 0.1 mol/L NaOH to various values in the range of 2-6. Then, 25 mg of PPPDA and PPPDA/Chi were separately added to each flask. The solutions were agitated by magnetic stirrer at 25 °C for 24 h to achieve the maximum adsorption. After equilibrium, a clear metal solution was taken and analyzed with atomic adsorption spectrophotometer to determine the amount of metal ion adsorbed on PPPDA and PPPDA/Chi composite. The effects of initial pH on the adsorption capacity and removal rate of Cu(II) ions were evaluated and the results are represented in Fig. 4, and the optimum pH values were determined. Studies beyond pH 6 were not attempted because the precipitation of the ions would likely be hydroxides [26]. At lower pH values, hydrogen ions occupy most of the adsorption sites on the surface of the adsorbents and consequently generate positively charged nitrogen and oxygen atoms. This results in a very low adsorption due to electrostatic repulsion between positive copper ion and positive functional groups on the surface of the polymer and its composite. However, increasing the pH of the solutions results in a decrease in the competition between hydrogen ions and Cu(II) ions, so, facilitating higher removal rate of Cu(II) ions. For PPPDA, the optimum pH value was 5 at which the removal rate was found to be 90% and adsorption capacity was 634 mg/g, and the removal rate increased gradually by increasing pH from 2 to 5 and then slightly decreased at pH 6. On the other hand, for PPPDA/Chi composite, the removal rate for the Cu(II) ion slightly increases at pH 2-4 and then sharp increase was observed at pH 4-5 followed by slight increase at pH 6, at this pH, the maximum removal rate was achieved (84.38%) and also the maximum adsorption capacity was 572 mg/g. PPPDA showed higher removal rate and adsorption capacity than those for PPPDA/Chi composite and this may be attributed to hydrogen bonding in the composite.
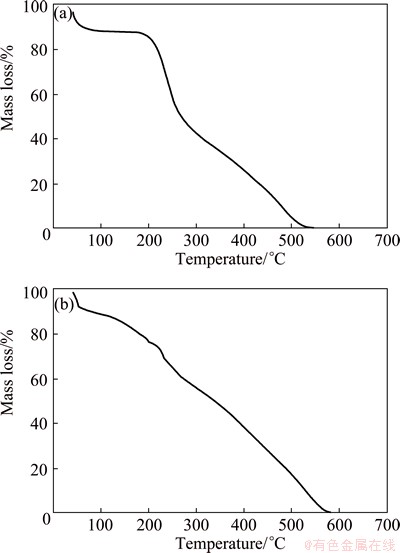
Fig. 3 TGA of PPPDA (a) and PPPDA /Chi composite (b)
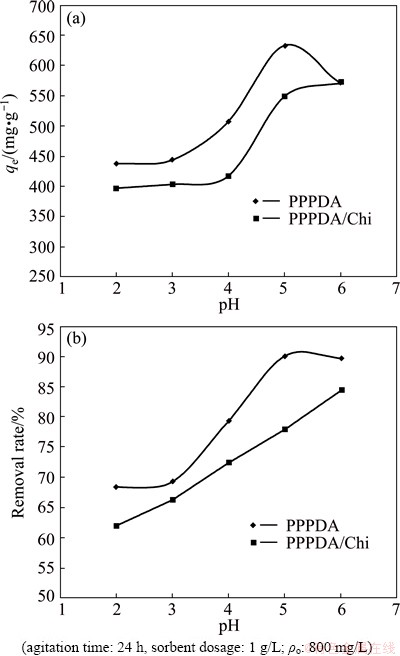
Fig. 4 Effect of pH on adsorption capacity (a) and removal rate (b) of Cu(II) ions by PPPDA and PPPDA/Chi composite
3.3 Effect of agitation time and sorbent dosage
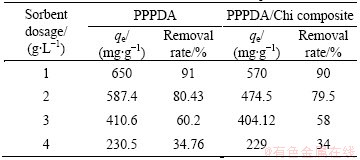
The effects of the agitation time and sorbent dosage on the adsorption capacity and removal rate of Cu(II) ions by PPPDA and PPPDA/Chi composite were studied and the results are presented in Tables 1 and 2. The experiments were examined at pH 5 for PPPDA and 6 for PPPDA/Chi composite, initial Cu(II) concentration 800 mg/L, and sorbent concentration 1 g/L. Equilibration time was reached when maximum removal rates were obtained and this was achieved after 360 min for both sorbents. After this equilibrium period, the amount of metal adsorbed did not change significantly with time. The removal efficiency increases rapidly at initial stage due to availability of active binding sites on the sorbent, and with gradual occupancy of these sites, the sorption became less efficient in the later stages [49]. The removal efficiency of Cu(II) by PPPDA and PPPDA/Chi composite at sorbent dosage ranging from 1-4 g/L was studied. The experiments were carried out at pH 5 for PPPDA and 6 for PPPDA/Chi composite, initial Cu(II) concentration 800 mg/L, for 360 min. From these results, it was observed that the adsorption capacity and removal rate decrease with increasing sorbent dosage from 1 to 4 g/L. This is mainly because the adsorption equilibrium may not have attained with the sorbent dosage more than 1 g/L. The optimum sorbent dosage was found to be 1 g/L for both sorbents.
Table 1 Effect of agitation time and sorbent dosage on adsorption capacity and removal rate for PPPDA and PPPDA/Chi composite
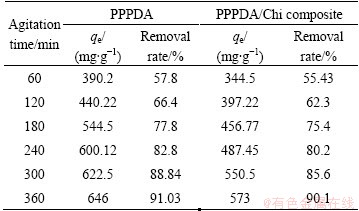
Table 2 Effect of sorbent dosage on adsorption capacity and removal rate for PPPDA and PPPDA/Chi composite
3.4 Adsorption isotherm studies
3.4.1 Freundlich isotherm
The Freundlich isotherm equation [50] is presented as follows:
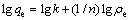 (2)
(2)
This isotherm represents relationship between the adsorption capacity (qe) and the concentration of Cu(II) ion (ρe) at equilibrium, where n and k are isotherm constants of Freundlich equation incorporating intensity and relative adsorption capacity, respectively. These equilibrium constants are evaluated from the slope and the intercept, respectively, of the linear plot of lg qe versus lg ρe based on the experimental data. The Freundlich equation can be linearized in logarithmic form for the determination of the Freundlich constants. The results are presented in Fig. 5 and the constant values are listed in Table 3. The values of 1/n lying between 0 and 1 confirmed the favorable conditions for adsorption. The higher R2 values obtained for both the sorbents indicate the applicability of Freundlich isotherm.
3.4.2 Langmuir isotherm
The Langmuir adsorption isotherm found successful applications in many other real adsorption processes of monolayer adsorption. Langmuir adsorption model depends on the assumption that intermolecular forces decrease rapidly with distance and consequently predicts the existence of monolayer coverage of the adsorbate on the outer surface of the adsorbent. Langmuir isotherm equation [51] model is presented as
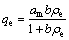 (3)
(3)
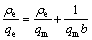 (4)
(4)
where qm is the amount of adsorbate at complete monolayer coverage, which gives the maximum sorption capacity of sorbent; and b is the Langmuir isotherm constant which reflects quantitatively the affinity between PPPDA or PPPDA/Chi composite and Cu(II) ions and calculated from the slope and intercept of the plot of ρe/qe vs ρe as shown in Fig. 5, and the values are given in Table 3. The isotherm equation further assumes that adsorption takes place at specific homogeneous sites within the adsorbent and when a metal ion occupies a site, no further adsorption can take place at that site. Moreover, the Langmuir equation is based on the assumption of a structurally homogeneous adsorbent where all adsorption sites are identical and energetically equivalent. The higher R2 values of Freundlich isotherm over Langmuir isotherm for both sorbents indicate the suitability of Freundlich isotherm.
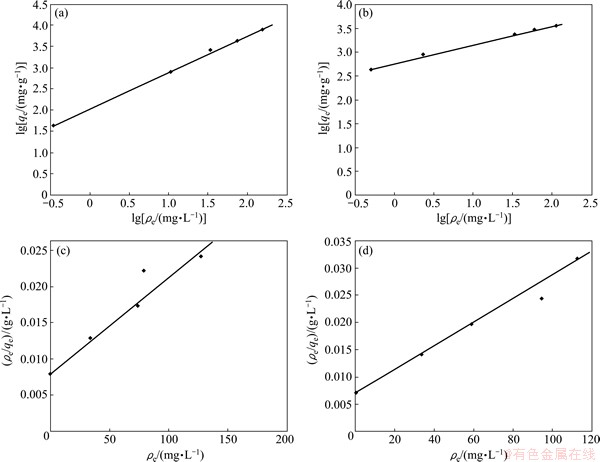
Fig. 5 Freundlich and Langmuir isotherms of PPPDA (a, c) and PPPDA/Chi composite (b, d)
Table 3 Freundlich and Langmuir isotherms constants
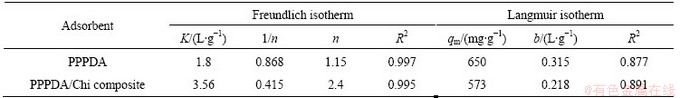
3.5 Sorption kinetic studies
The kinetic of adsorption results can be analyzed with various adsorption kinetic models to reveal the control factors in the adsorption process. The pseudo- first order model equation [52] is presented by
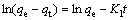 (5)
(5)
where qe and qt are the amounts of metal ion adsorbed per unit mass of adsorbent at equilibrium and at any time t, respectively. By plotting ln(qe-qt) versus t, the first-order adsorption rate constant K1 can be calculated.
The pseudo-second order model [53] is expressed by
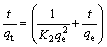 (6)
(6)
A plot of t/qt against t provides pseudo-second order adsorption rate constant K2 value from the slopes and intercepts.
The intraparticle diffusion model [54] is a relationship between the amount of adsorbed metal ion and the square root of time which is expressed as
qt=Kidt0.5 (7)
where Kid is initial rate of the intraparticle diffusion. The value of Kid can be obtained from the slope of the plot of qt versus t0.5.
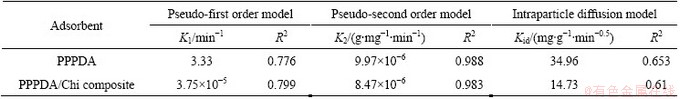
The three models are shown in Fig. 6 and the corresponding parameters of equations were determined by linear regression and represented in Table 4. From the correlation coefficient (R2) values, it was found that the pseudo-second order model is the most appropriate one to describe the adsorption kinetics of Cu(II) ions onto PPPDA and PPPDA/Chi composite and the chemisorption is the rate controlling mechanism. For the intraparticle diffusion plot, the curved part is due to boundary layer effect and the linear part indicates intraparticle or pore diffusion. This suggests that the adsorption process proceeds by surface adsorption and intraparticle diffusion.
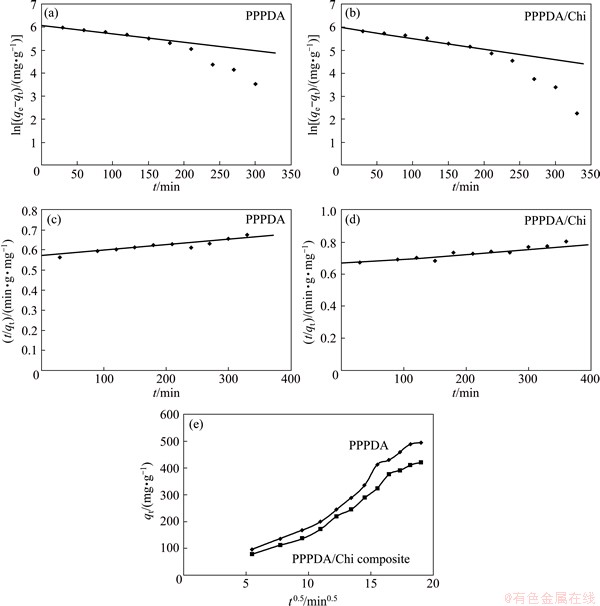
Fig. 6 First-order (a, b), second-order (c, d) and intraparticle diffusion models (e) for PPPDA and PPPDA/Chi composites
3.6 Mechanism of copper sorption
The mechanism of copper removal by PPPDA and PPPDA/Chi composite was studied on the basis of adsorption, ion-exchange and chelation. Also, the mechanism of sorption depends on the chemical structure of PPPDA and its composite. PPPDA has lone pair on N atom. So, the removal of copper ion may be by chelation mechanism to form complex layer covering the surface of PPPDA. This was also confirmed by the observed shift in the peak corresponding to NH of PPPDA. For PPPDA/Chi composite besides to NH of PPPDA, chitosan has amino and hydroxyl groups which are chelate Cu(II) ions. The proposed mechanism is shown in Scheme 2.
3.7 Desorption and reusability studies
From economical point of view, it is important to study the desorption of Cu(II) ion from the studied sorbents (PPPDA and PPPDA/Chi composite) and reuse them. For this purpose, 0.01 mol/L EDTA, 0.01 mol/L HCl and 0.01 mol/L HNO3 were used as desorbing agents. It was found that EDTA showed the highest desorption efficiency (98.6% for PPPDA and 98.3% for PPPDA/Chi) followed by HCl (90.3% for PPPDA and 90.2% for PPPDA/Chi) and the lowest values were obtained with HNO3 as desorption agent (84.6% for PPPDA and 84% for PPPDA/Chi). The reusability of PPPDA and PPPDA/Chi was examined for four adsorption-desorption cycles. It was found that the adsorption capacity for both PPPDA and PPPDA/Chi composite decreases gradually with increasing cycle number, as shown in Table 5. It can be noticed that there is no significant difference of desorption efficiency between PPPDA and PPPDA/Chi composite.
Table 4 Kinetics parameters for Cu(II) adsorption onto PPPDA and PPPDA/Chi composite
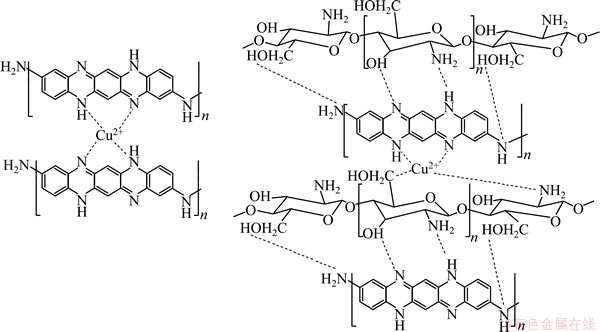
Scheme 2 Proposed mechanism for copper sorption
Table 5 Adsorption-desorption cycles for PPPDA and PPPDA/ Chi composite
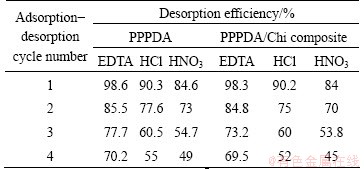
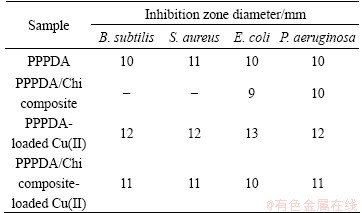
Table 6 Antibacterial activity of PPPDA, PPPDA/Chi composite, PPPDA-loaded Cu(II) and PPPDA/Chi composite- loaded Cu(II)
3.8 Antibacterial activity
For this study, B. subtilis and S. aureus (as Gram- positive bacteria strains), E. coli and P. aeruginosa (as Gram-negative bacteria strains) were employed. The antibacterial activities of PPPDA, PPPDA/Chi composite and their complexes with copper were investigated by recording the diameter of inhibition zone and the results are given in Table 6. The copper complexes of polymeric ligand (PPPDA) and its composite with chitosan (PPPDA/Chi) showed higher antibacterial activities than free ligands and this is in agreement with the previous studies [55]. The PPPDA and PPPDA/Chi-copper complexes have lipophilic character due to partial sharing of positive charge of copper metal, so, the delocalization of π-electrons increases for polymer- metal complexes. This could enhance their lipophilicity and consequently their ability to penetrate lipid layer of bacterial cell membrane increased. Moreover, polymer-metal complexes cause blocking of cell protein synthesis as a result of interaction with some functional groups on the bioactive protease such as sulfhydryl group (—SH), amino group (—NH2), hydroxyl group (—OH) to change the structure and performance of protein so as to die in the absence of normal balanced metabolism [56]. PPPDA/Chi-copper complex showed antibacterial activity against Gram-negative bacteria but it had no effect towards Gram-positive bacteria.
4 Conclusions
PPPDA and PPPDA/Chi composites were prepared, FT-IR spectra confirmed polymer, polymer composite formation and also their copper complexes. Some shifts in frequencies for the peaks corresponding to —OH and NH2 in Chi composite and —NH of PPPDA were observed. SEM showed incorporation of PPPDA agglomerates between flakes of chitosan and confirmed the copper loading for both studied sorbents. TGA indicates higher thermal stability of PPPDA than its composite. Maximum adsorption capacity for both sorbents was achieved at pH 5.0 for PPPDA and 6.0 for PPPDA/Chi composite, agitation time 360 min and sorbent dosage 1 g/L. The adsorption of copper onto PPPDA and PPPDA/Chi composite follows Freundlich isotherm. The adsorption kinetics is well described by the pseudo-second order equation. Removal of copper by PPPDA and PPPDA/Chi composite was governed by adsorption and chelation mechanism. The Cu2+-loaded PPPDA and Cu2+-loaded PPPDA/Chi composite could be regenerated efficiently with 0.01 mol/L EDTA solution and reused repeatedly for Cu(II) adsorption for as many as four cycles. The Cu(II) complexes of PPPDA and PPPDA/Chi composite showed higher antibacterial properties against B. subtilis and S. aureus (as Gram- positive bacteria strains), E. coli and P. aeruginosa (as Gram-negative bacteria strains) than free ones due to enhancement of electrostatic charge.
Acknowledgments
The authors would like to thank National Research Centre (NRC) for its technical support.
References
[1] YAN H, YANG L, YANG Z, YANG H, LI A, CHENG R. Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions [J]. Journal of Hazardous Materials, 2012, 229-230(7): 371-380.
[2] FU H, KOBAYASHI T. Self-assembly functionalized membranes with chitosan microsphere/polyacrylic acid layers and its application for metal ion removal [J]. Journal of Materials Science, 2010, 45(24): 6694-6700.
[3] DAI J, YAN H, YANG H, CHENG R S. Simple method for preparation of chitosan/poly(acrylic acid) blending hydrogel beads and adsorption of copper(II) from aqueous solutions [J]. Chemical Engineering Journal, 2010, 165(1): 240-249.
[4] LIU X W, HU Q Y, FANG Z, ZHANG X J, ZHANG B B. Magnetic chitosan nanocomposites: A useful recyclable tool for heavy metal ion removal [J]. Langmuir, 2009, 25 (1): 3-8.
[5] LPD M, FVM A, MF C, KAN C C, TSAI W C, WAN M W. Adsorptive removal of Cu(II) from aqueous solutions using non-crosslinked and crosslinked chitosan-coated bentonite beads [J]. Desalination, 2011, 275(1-3): 154-159.
[6] FUTALAN C M, KAN C C, DALIDA M L, PASCUA C, WAN M W. Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite [J]. Carbohydrate Polymers, 2011, 83(2): 697-704.
[7] FUTALAN C M, KAN C C, DALIDA M L, HSIEN K J, PASCUA C, WAN M W. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite [J]. Carbohydrate Polymers, 2010, 83(2): 528-536.
[8] NGAH W S W, FATINATHAN S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads [J]. Chemical Engineering Journal, 2008, 143(1-3): 62-72.
[9] HUANG G, YANG C, ZHANG K, SHI J. Adsorptive removal of copper ions from aqueous solution using cross-linked magnetic chitosan beads [J]. Chinese Journal of Chemical Engineering, 2009, 17(6): 960-966.
[10] RAO M M, RAMESH A, RAO G P C, SESHAIAH K. Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls [J]. Journal of Hazardous Materials B, 2006, 129(1-3): 123-129.
[11] TIAN Y, LI Z Q, XU H F, YANG F L. Comparison on electroreduction of Cu(II) using polypyrrole and stainless steel electrodes [J]. Separation and Purification Technology, 2008, 63(2): 334-340.
[12] HUANG C H, CHEN L K, YANG C L. Effect of anions on electrochemical coagulation for cadmium removal [J]. Separation and Purification Technology, 2009, 65 (2): 137-146.
[13] RENGARAJ S, YEON K H, MOON S H. Removal of chromium from water and wastewater by ion exchange resins [J]. Journal of Hazardous Materials B, 2001, 87(1-3): 273-287.
[14] KAMBLE S B, MARATHE K V. Membrane characteristics and fouling study in MEUF for the removal of chromate anions from aqueous streams [J]. Separation and Purification Technology, 2005, 40(12): 3051-3070.
[15] YANG H, YUAN B, LU Y B, CHENG R S. Preparation of magnetic chitosan micro-spheres and its applications in wastewater treatment [J]. Science in China Series B: Chemistry, 2009, 52: 249-256.
[16] LI N, BAI R B. Highly enhanced adsorption of lead ions on chitosan granules functionalized with poly(acrylic acid) [J]. Industrial & Engineering Chemistry Research, 2006, 45: 7897-7904.
[17] RINAUDO M. Chitin and chitosan: Properties and applications [J]. Progress in Polymer Science, 2006, 31(7): 603-632.
[18] SCHMUHL R, KRIEG H M, KEIZER K. Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetics and equilibrium studies [J]. Water SA, 2001, 27(1): 1-7.
[19] WAN M W, WANG C C, CHEN C M. The adsorption study of copper removal by Chitosan-coated sludge derived from water treatment [J]. International Journal of Environmental Science and Development, 2013, 4(5): 545-551.
[20] SVETLANA V, MYKHAYLO B, GANNA C, BRYAN F. Removal of copper(II) from aqueous solutions by chitosan adsorption [J]. Separation Science and Technology, 2005, 40(8): 1749-1759.
[21] SRINIVASA R P, VIJAYA Y, VEERA M B, KRISHNAIAH A. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads [J]. Bioresource Technology, 2009, 100(1): 194-199.
[22] MUNIYAPPAN R G, KOUSALYAB G N, NATRAYASAMY V, MEENAKSHIA S. Sorption behaviour of copper on chemically modified chitosan beads from aqueous solution [J]. Carbohydrate Polymers, 2011, 83(3): 1082-1087.
[23] KANNAMBA B, REDDY K L, APPARAO B V. Removal of Cu(II) from aqueous solutions using chemically modified chitosan [J]. Journal of Hazardous Materials, 2010, 175(1-3): 939-948.
[24] AHMED J M A, ZYAD H J A, HUSSEIN I A, ALI M A A, DHAFIR T A A. Synthesis, characterization of acrylamide grafted chitosan and its use in removal of copper(II) ions from water [J]. Carbohydrate Polymers, 2011, 83(2): 495-500.
[25] WANG X H, ZHENG Y, WANG A Q. Fast removal of copper ions from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite composites [J]. Journal of Hazardous Materials, 2009, 168(1): 970-977.
[26] NGAH W S W, TEONG L C, TOH R H, HANAFIAH M A K M. Utilization of chitosan–zeolite composite in the removal of Cu(II) from aqueous solution: Adsorption, desorption and fixed bed column studies [J]. Chemical Engineering Journal, 2012, 209: 46-53.
[27] ROHWERDER M, MICHALIK A. Conducting polymers for corrosion protection: What makes the difference between failure and success [J]. Electrochimica Acta, 2007, 53(3): 1300-1313.
[28] LAI C Z, FIERKE M A, STEIN A, BUHLMANN P. Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact [J]. Analytical Chemistry, 2007, 79(12): 4621-4626.
[29] HUANG M R, PENG Q Y, LI X G. Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles [J]. Chemical European Journal, 2006, 12(16): 4341-4350.
[30] NASALSKA A, SKOMPSKA M. Removal of toxic chromate ions by the film of poly(1,8-diaminonapthalene) [J]. Journal of Applied Electrochemistry, 2003, 33(1): 113-119.
[31]  B, SKOMPSKA M, JACKOWSKA K. Sensitivity of poly 1,8-diaminonapthalene to heavy metal ions–Electrochemical and vibrational spectra studies [J]. Journal of Electroanalytical Chemistry, 1997, 433(1): 41-48.
B, SKOMPSKA M, JACKOWSKA K. Sensitivity of poly 1,8-diaminonapthalene to heavy metal ions–Electrochemical and vibrational spectra studies [J]. Journal of Electroanalytical Chemistry, 1997, 433(1): 41-48.
[32] LI X G, HUANG M R, LI S X. Facile synthesis of poly (1,8- diaminonaphthalene) microparticles with a very high silver-ion adsorbability by a chemical oxidative polymerization [J]. Acta Materialia, 2004, 52(18): 5363-5374.
[33] LI X G, LIU R, HUANG M R. Facile synthesis and highly reactive silver ion adsorption of novel microparticles of sulfonated diphenylamine and diaminonaphthalene copolymers [J]. Chemistry of Materials, 2005, 17(22): 5411-5419.
[34] HUANG M R, LU H J, LI X G. Efficient multicyclic sorption and desorption of lead ions on facilely prepared poly (m-phenylenediamine) particles with extremely strong chemoresistance [J]. Journal of Colloids and Interface Science, 2007, 313(1): 72-79.
[35] SU Z, ZHANG L, CHAI L, YU W, WANG H, SHI Y. Methanol-induced formation of 1D poly(m-phenylenediamine) by conventional chemical oxidativepolymerizationexhibiting superior Ag+ adsorptionability [J]. RSC Advance, 2013, 3: 8660-8665.
[36] ZHANG L, CHAI L, LIU J, WANG H, YU W, SANG P. pH manipulation: A facile method for lowering oxidation state and keeping good yield of poly(m-phenylenediamine) and its powerful Ag+ adsorption ability [J]. Langmuir,2011,27(22): 13729-13738.
[37] YU W, ZHANG L, WANG H, CHAI L. Adsorption of Cr(VI) using synthetic poly(m-phenylenediamine) [J]. Journal of Hazardous Materials, 2013, 260: 789-795.
[38] SANG Pei-yun, WANG Yun-yan, ZHANG Li-yuan, CHAI Li-yuan, WANG Hai-ying. Effective adsorption of sulfate ions with poly(m-phenylenediamine) in aqueous solution and its adsorption mechanism [J]. Transactions of Nonferrous Metals Society of China, 2013, 23 (1): 243-252.
[39] VIEL P, PALACIN S, DESCOURS F, BUREAU C, DERF F L, LYSKAWA J, SALLE M. Electropolymerized poly-4-vinylpyridine for removal of copper from wastewater [J]. Applied Surface Science, 2003, 212-213: 792-796.
[40] MANSOUR M S, OSSMAN M E, FARAG H A. Removal of Cd (II) ion from wastewater by adsorption onto polyaniline coated on sawdust [J]. Desalination, 2011, 272(1-3): 301-305.
[41] MOHAMAD O, HOSSEIN E, REZA K, MOHSEN G. Study of the removal of Zn(II) from aqueous solution using polypyrrole nanocomposite [J]. Desalination, 2011, 271(1): 248-256.
[42] WEBER W J, MORRIS J C. Kinetics of adsorption on carbon from solution [J]. Journal of the Sanitary Engineering Division, American Society of Civil Engineers, 1963, 89(17): 31-59.
[43] ZHENG L Y, ZHU J F. Study on antimicrobial activity of chitosan with different molecular weights [J]. Carbohydrate Polymers, 2003, 54(4): 527-530.
[44] SUFIA H. Removal of chromium hexavalent ion from aqueous solutions using biopolymer chitosan coated with poly 3-methyl thiophene polymer [J]. Journal of Hazardous Materials, 2010, 181(1-3): 474-479.
[45] IGBERASE E, OSIFO P, OFOMAJA A. The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueous solution: Equilibrium, kinetic and desorption studies [J]. Journal of Environmental Chemical Engineering, 2014, 2(1): 362-369.
[46] RICARDO H S, DANIELA C F, RICHARD L, MARCIA L A T, GUSTAVO M N. Structure of chemically prepared poly-(para- phenylenediamine) investigated by spectroscopic techniques [J]. Polymer, 2009, 50(25): 6043-6048.
[47] AYSE G Y, AYSEGUL U, VENKAT R B. Substituted polyaniline/chitosan composites: Synthesis and characterization [J]. Carbohydrate Polymers, 2009, 75(3): 448-453.
[48] KARTHIKEYAN M, KUMAR K K S, ELANGO K P. Batch sorption studies on the removal of fluoride ions from water using eco-friendly conducting polymer/bio-polymer composites [J]. Desalination, 2011, 267(1): 49-56.
[49] NOMANBHAY S M, PALANISAMY K. Removal of heavy metals from industrial wastewater using chitosan coated oil palm shell charcoal [J]. Electronic Journal of Biotechnology, 2004, 8(1): 43-53.
[50] FREUNDLICH H M F.  [J]. Zeitschrift für Physikalische Chemie A, 1906, 57: 385-470. (in Germany)
[J]. Zeitschrift für Physikalische Chemie A, 1906, 57: 385-470. (in Germany)
[51] LANGMUIR I. The constitution and fundamental properties of solids and liquids, Part I: Solids [J]. Journal of American Chemical Society, 1916, 38: 2221-2295.
[52] LAGERGREN S. Zur theorie der sogenannten adsorption geloster stoffe [J]. Kungliga Svenska Vetenskapsakademiens, 1898, 24(4): 1-39. (in Germany)
[53] HO Y S, MCKAY G. Pseudo-second order model for sorption processes [J]. Process Biochemistry, 1999, 34(5): 451-465.
[54] GIZDAVIC-NIKOLAIDIS M R, BENNETT J R, SWIFT S, EASTEAL A J, AMBROSE M. Broadspectrum antimicrobial activity of functionalized polyanilines [J]. Acta Biomaterialia, 2011, 7(12): 4204-4209.
[55] RAMAN N, KULANDAISAMY A, SHUNMUGASUNDARAM A, JEYASUBRAMANIAN K. Synthesis, spectral, redox and antimicrobial activities of Schiff base complexes derived from 1-phenyl-2,3- dimethyl-4-aminopyrazol-5-one and acetoacetanilide [J]. Transition Metal Chemistry, 2001, 26(1-2): 131-135.
[56] DROVAL G, ARANBERRI I,  L, IVANOV E, DIMITROVA E, KOTSILKOVA R, VERELST M, DEXPERT-GHYS J. Thermal and rheological characterization of antibacterial nanocomposites: Poly(amide) 6 and low-density poly(ethylene) filled with zinc oxide [J]. Journal of Thermoplastic Composites, 2014, 27(2): 1-8.
L, IVANOV E, DIMITROVA E, KOTSILKOVA R, VERELST M, DEXPERT-GHYS J. Thermal and rheological characterization of antibacterial nanocomposites: Poly(amide) 6 and low-density poly(ethylene) filled with zinc oxide [J]. Journal of Thermoplastic Composites, 2014, 27(2): 1-8.
N. A. ABDELWAHAB1, E. A. AL-ASHKAR2, M. A. ABD EL-GHAFFAR1
1. Polymers and Pigments Department, National Research Centre, 33 El Bohouth St. Dokki, Giza, P. O. 12622, Egypt;
2. Spectroscopy Department, Physics Division, National Research Centre, 33 El Bohouth St. Dokki, Giza, P. O. 12622, Egypt
摘 要:利用过硫酸铵作为氧化剂,在壳聚糖(Chi)中用对苯二胺(PPPDA)原位化学氧化聚合反应制备聚(对苯二胺)/壳聚糖复合材料。在添加铜前后,利用FT-IR光谱和SEM表征对苯二胺和对苯二胺/壳聚糖复合材料。利用振荡吸附法,采用1 g/L对苯二胺和对苯二胺/壳聚糖复合材料,在PPPDA和PPPDA/Chi复合材料的pH分别为5.0和6.0的条件下,吸附360 min,可以得到最大的铜去除量。PPPDA显示最大吸附量为650 mg/g,而复合材料的吸附量达到 573 mg/g。实验数值与Freundlich等温方程和伪二阶动力学模型吻合良好。含铜的对苯二胺及其复合材料可以被有效地再利用4个循环。相对于无吸附的状态,吸附获得的铜具有高的革兰氏阳性和革兰氏阴性菌抑菌效果。
关键词:铜去除;吸附;聚(对苯二胺)/壳聚糖复合材料;动力学;等温线
(Edited by Xiang-qun LI)
Corresponding author: N. A. ABDELWAHAB; Tel: +20-2-01223488928; E-mail: nor_5020@yahoo.com
DOI: 10.1016/S1003-6326(15)64025-0

