
Synthesis and electrochemical performances of
LiNi0.6Co0.2Mn0.2O2 cathode materials
ZHONG Sheng-kui(钟胜奎)1, 2, LI Wei(李 伟)1, LI Yan-hong(李艳红)3,
ZUO Zheng-guang(邹正光)1, 2, TANG Xin(唐 鑫)1, 2
1. Department of Material and Chemistry, Guilin University of Technology, Guilin 541004, China;
2. Key Laboratory of New Processing Technology for Nonferrous Metals and Materials,
Ministry of Education, Guilin University of Technology, Guilin 541004, China
Received 10 August 2009; accepted 15 September 2009
Abstract: LiNi0.6Co0.2Mn0.2O2 was prepared from LiOH·H2O and MCO3 (M=Ni, Co, Mn) by co-precipitation and subsequent heating. XRD, SEM and electrochemical measurements were used to examine the structure, morphology and electrochemical characteristics, respectively. LiNi0.6Co0.2Mn0.2O2 samples show excellent electrochemical performances. The optimum sintering temperature and sintering time are 850 ℃ and 20 h, respectively. The LiNi0.6Co0.2Mn0.2O2 shows the discharge capacity of 148 mA?h/g in the range of 3.0-4.3 V at the first cycle, and the discharge capacity remains 136 mA?h/g after 30 cycles. The carbonate co-precipitation method is suitable for the preparation of LiNi0.6Co0.2Mn0.2O2 cathode materials with good electrochemical performance for lithium ion batteries.
Key words: lithium ion batteries; LiNi0.6Co0.2Mn0.2O2; cyclic voltammogram (CV)
1 Introduction
Research on cathode materials of lithium-ion battery was mainly concentrated in the transition metal oxides of layered structure such as LiCoO2, LiNiO2, LiMnO2 and their derivatives. LiCoO2 is one of the first commercial lithium-ion battery cathode materials, but its application is limited because of the scarce cobalt resources, high prices and a certain degree of toxicity [1-3]. It is hard to prepare LiNiO2 and the crystal structure will change during the charge-discharge process, resulting in much attenuation of its capacity[4-5]. LiMnO2 with layered structure has high theoretical specific capacity of 285 mA?h/g. However, during the process of charging and discharging, the structure easily changes to the spinel structure, resulting in fast attenuation of capacity[6]. Recently, intensive effort has been directed towards the development of LiNixCoyMn1-x-yO2 (e.g. LiNi1/3Co1/3Mn1/3O2) as possible replacement for LiCoO2[7-11]. It was reported that the capacity obtained for LiNixCoyMn1-x-yO2 was dependent upon composition, synthesis technology and charge/ discharge voltage. The first discharge specific capacity reported is often more than 180 mA?h/g with good cycling stability in the first 30 or 50 cycles when being charged to 4.6 V (vs Li/Li+). However, the cycling stability of this kind of material in more cycles has been less discussed because of the appearance of Li-branch on the surface of Li anode after some cycles.
In this work, a two-stage technique consisting of co-precipitation and high temperature sintering was employed to prepare the layered LiNi0.6Co0.2Mn0.2O2 cathode material. Effects of the heating temperature and time on structure and electrochemical properties are tested.
2 Experimental
2.1 Preparation of cathode materials
The precursor (Ni0.6Co0.2Mn0.2)CO3 was prepared by the co-precipitation method. Cobalt acetate (Co(CH3-COO)2), nickel acetate (Ni(CH3COO)2), and manganese acetate (Mn(CH3COO)2) were dissolved in deionized water with n(Co)?n(Ni)?n(Mn) of 2?6?2 to obtain a mixture. Then, sodium carbonate (Na2CO3) solution was added into the mixture with 1?1(volume ratio) of NH3-to-H2O to obtain a solution with the pH value of about 11. It was subsequently heated at 60 ℃ for 3 h to react, then was rested for about 2.5 h. It was calcined at 100 ℃ for 4 h to obtain the precursor (Ni0.6Co0.2- Mn0.2)CO3. Then, the dried (Ni0.6Co0.2Mn0.2)- CO3 were ground together with LiOH·H2O in the molar ratio n(CO32-)?n(Li+) of 1?2. The powders were subsequently annealed at a temperature from 750 ℃ to 950 ℃ in air for 20 h followed by a natural cooling to obtain LiNi0.6Co0.2Mn0.2O2 powers. To investigate the effect of annealing time, the powders were further annealed at 850 ℃ for different time of 10, 20 and 30 h, respectively.
2.2 Sample characterization
The powder X-ray diffraction (XRD, X Pert-Pro) measurement using Cu Kα radiation was employed at room temperature to identify the crystalline phase of the synthesized materials. The particle size and morphology of the LiNi0.6Co0.2Mn0.2O2 powders were observed by scanning electron microscope (JEOL, GSM-6380LV) with an accelerating voltage of 15 kV.
2.3 Electrochemical performances evaluation
The electrochemical characterizations were performed using CR2025 coin-type cell. For positive electrode fabrication, the prepared powders were mixed with 10%(mass fraction) carbon black and 10%(mass fraction) polyvinylidene fluoride in N-methyl pyrrolidinone until slurry was obtained. And then, the blended slurries were pasted onto an aluminum current collector, and the electrode was dried at 120 ℃ for 10 h in vacuum. The test cell consisted of the positive electrode and lithium foil negative electrode separated by a porous polypropylene film, with 1 mol/L LiPF6 in EC?EMC?DMC (1?1?1 in volume ratio) as the electrolyte. The assembly of the cells was carried out in a dry Ar-filled glove box. The cells were charged and discharged over a voltage range from 3.0 V to 4.3 V versus Li/Li+ at room temperature. Cyclic voltammograms were tested in the three-electrode system using metallic foils as both counter and reference electrodes at a scanning rate of 0.1 mV/s in the voltage range of 3.0-4.5 V.
3 Results and discussion
3.1 XRD patterns of synthesized LiNi0.6Co0.2Mn0.2O2 samples
Fig.1 shows the XRD patterns of prepared LiNi0.6- Co0.2Mn0.2O2 powders heated at 750, 850 and 950 ℃. All peaks are indexed on the basis of the α-NaFeO2 structure(R3m). It is clear that all samples have a typical hexagonal layered structure because both the (006)/(102) and the (018)/(110) are well resolved, especially when the heating temperature is above 850 ℃.
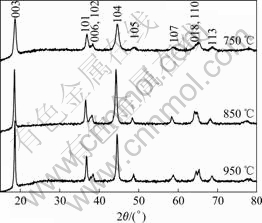
Fig.1 XRD patterns for LiNi0.6Co0.2Mn0.2O2 powders heated at 750 ℃, 850 ℃ and 950 ℃
The X-ray diffraction patterns of LiNi0.6Co0.2- Mn0.2O2 powders annealed for different time from 10 to 30 h at 850 ℃ are shown in Fig.2. The XRD patterns of all samples are consistent with single phase of α-NaFeO2 structure. It is also clear that all samples have a typical hexagonal layered structure.
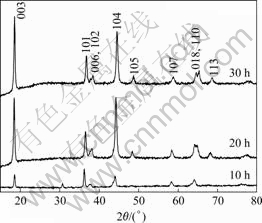
Fig.2 XRD patterns for LiNi0.6Co0.2Mn0.2O2 powders annealed at 850 ℃ for different time
3.2 SEM images of synthesized LiNi0.6Co0.2Mn0.2O2 samples
Fig.3 shows the scanning electron microscopy (SEM) images of the LiNi0.6Co0.2Mn0.2O2 powders annealed at 750, 850 and 950 ℃ for 20 h. With increasing calcination temperature, the primary particle size gradually increases. It could be noticed that LiNi0.6Co0.2Mn0.2O2 powders calcined at 850 ℃ exhibit relatively uniform particle size, as shown in Fig.3(b), while LiNi0.6Co0.2Mn0.2O2 powders annealed at 750 and 950 ℃ consist of some primary particles and larger secondary ones. This indicates that raising the calcined temperature not only increases particle size, but also affects the uniformity of the materials.
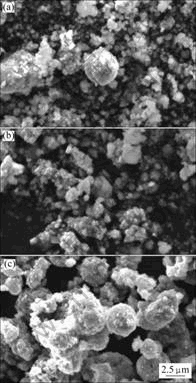
Fig.3 SEM images of LiNi0.6Co0.2Mn0.2O2 powders annealed at 750 ℃(a), 850 ℃(b) and 950 ℃(c) for 20 h
Fig.4 shows the SEM images of the LiNi0.6Co0.2Mn0.2O2 powders annealed at 850 ℃ for 10, 20 and 30 h. With increasing calcination time, the primary particle size gradually increases. It could be noticed that LiNi0.6Co0.2 Mn0.2O2 powders calcined for 20 h exhibit relatively uniform particle size, as shown in Fig.4(b), while LiNi0.6Co0.2 Mn0.2O2 powders annealed for 10 and 30 h consist of some primary particles and larger secondary ones. This indicates that raising the calcined time not only increases particle size, but also affects the uniformity of the materials.

Fig.4 SEM images of LiNi0.6Co0.2 Mn0.2O2 powders annealed at 850 ℃ for 10 h (a) , 20 h (b) and 30 h (c)
3.3 Electrochemical characteristics
Fig.5 shows the first charge-discharge curves of LiNi0.6Co0.2Mn0.2O2 cells calcined at various temperatures at the rate of 0.5C in the voltage range of 3.0-4.3 V at room temperature. As seen in Fig.5, the initial charge capacities for LiNi0.6Co0.2Mn0.2O2 calcined at of 750, 850 and 950 ℃ are about 156, 163 and 160 mA?h/g, and the discharge capacities are about 137, 148 and 142 mA?h/g, respectively. The corresponding coulombic efficiency is 87.8%, 90.7% and 88.7%, respectively. It is clearly seen that the electrode reaction reversibility is enhanced considerably when the heating temperature is selected as 850 ℃. This is because the homogenous particle of LiNi0.6Co0.2 Mn0.2O2 leads to a uniform depth of charge (DOC) of each particle, which increases the utilization of the material. Accordingly, the coulombic efficiency is increased.
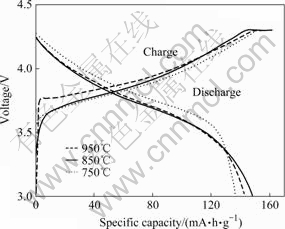
Fig.5 Initial charge-discharge curves of LiNi0.6Co0.2 Mn0.2O2 samples calcined at different temperatures
Fig.6 shows the specific discharge capacity with the cycle number for the LiNi0.6Co0.2Mn0.2O2 cell in the voltage range between 3.0 and 4.3 V. It can be seen that the 850 ℃-annealed LiNi0.6Co0.2Mn0.2O2 cell delivers a discharge capacity of 148 mA?h/g at 0.2C in the first cycle. For comparison, the initial specific discharge capacities are 137 and 142 mA?h/g for the samples annealed at 750 and 950 ℃, respectively. It is noticed that the initial specific discharge capacity increases with increasing the annealing temperature from 750 to 850 ℃, and decreases with further increasing the annealing temperature from 850 to 950 ℃. The following factors might cause this trend. First, the crystallinity of powders is one important factor affecting the electrochemical discharge capacity. The higher annealing temperature leads to higher crystallinity that helps to increase the electrode capacity. Second, the particle size of electrode material is also related to its electrochemical performance. During the first cycle, part of overall capacity is consumed to form a surface layer on the electrode particles. The larger particle size means a smaller specific surface area, resulting in lower capacity loss. Third, too high annealing temperature may be accompanied by the appearance of cation mixing and impurity phases. On the other hand, the irreversible capacity losses after 30 cycles are 12.5%, 8.2%, and 14.8%, respectively, as the annealing temperature increases from 750 to 950 ℃. The irreversibility comes mainly from the fact that a part of Ni3+ cannot be reduced to Ni2+ after the first cycle. Another main contributor is the formation of surface layer on the electrode material, especially for some material with small particle sizes.

Fig.6 Cycling performance of LiNi0.6Co0.2 Mn0.2O2 samples calcined at different temperatures
Fig.7 shows the first charge-discharge curves of LiNi0.6Co0.2Mn0.2O2 cells calcined at 850 ℃ for different time at the rate of 0.2C in the voltage range of 3.0-4.3 V at room temperature. As seen in Fig.7, it is clear that the initial charge capacities for LiNi0.6Co0.2 Mn0.2O2 calcined for 10, 20, 30 h are about 158, 163, 164 mA?h/g, and the discharge capacities are about 139, 148, 145 mA?h/g, respectively. The corresponding coulombic efficiencies are 87.9%, 90.7% and 88.4%, respectively. It is clearly seen that the electrode reaction reversibility is enhanced considerably when the heating time is 20 h.

Fig.7 Initial charge-discharge curves of LiNi0.6Co0.2 Mn0.2O2 samples calcined at 850 ℃ for different time
The electrochemical cycling performance of LiNi0.6Co0.2Mn0.2O2 samples is shown in Fig.8. It can be seen that the initial discharge capacities of LiNi0.6Co0.2Mn0.2O2 calcined for 10, 20 and 30 h are 139, 148, 145 mA?h/g, with the capacity losses of 10.8%, 8.2%, and 9.0% after 30 cycles, respectively. It is noticed that the initial specific discharge capacity increases with increasing the annealing time from 10 to 20 h and the capacity loss decreases with further increasing the annealing time from 10 to 20 h, then increases with increasing the annealing time from 20 to 30 h.
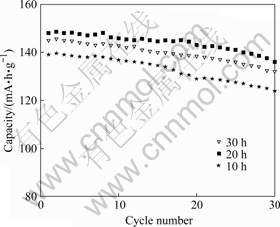
Fig.8 Cycling performance of LiNi0.6Co0.2 Mn0.2O2 samples calcined at 850 ℃ for different time
The initial cyclic voltammogram (CV) curves for LiNi0.6Co0.2 Mn0.2O2 electrode at a scanning rate of 0.1 mV/s is shown in Fig.9. It can be seen that the oxidation peak of LiNi0.6Co0.2Mn0.2O2 is observed at around 4.05 V, coupled with the reduction peak at 3.67 V. The appearance of only one couple of peak in the LiNi0.6Co0.2Mn0.2O2/Li cell between 3.0 and 4.6 V means that no structural transitions exist from hexagonal to monoclinic.
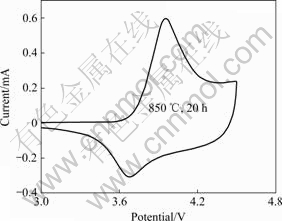
Fig.9 CV curves of LiNi0.6Co0.2 Mn0.2O2 electrode in first cycle
4 Conclusions
1) LiNi0.6Co0.2Mn0.2O2 cathode materials are prepared by co-precipitation and subsequent heating. XRD and SEM analyses show that the structures and morphology of the LiNi0.6Co0.2Mn0.2O2 samples heated at different temperatures and time are different, which results in different electrochemical performances.
2) The LiNi0.6Co0.2Mn0.2O2 sample annealed at 850 ℃ for 20 h shows the best electrochemical properties with the first specific discharge capacity of 148 mA?h/g in the range of 3.0-4.3 V with the capacity losses of 8.2% after 30 cycles.
3) LiNi0.6Co0.2Mn0.2O2 is tested to be a promising cathode material for lithium ion batteries.
References
[1] KOKSBANG R, BARKER J, SHI H. Cathode materials for lithium rocking chair batteries [J]. Solid State Ionics, 1996, 84: 1-21.
[2] RANDOLPH A L, MARCUS J P, ESTHER S T, KENNETH J T. A study of the overcharge reaction of lithium-ion batteries [J]. J Power Sources, 2001, 97/98: 681-684.
[3] WON S Y, KWANG B K. Synthesis of LiCoO2 using acrylic acid and its electrochemical properties for Li secondary batteries [J]. J Power Sources, 1999, 81/82: 517-523.
[4] ARAI H, OKADA S, SAKURAI Y, YAMAKI J I. Reversibility of LiNiO2 cathode [J]. Solid State Ionics, 1997, 95(3/4): 275-282.
[5] ZHONG S W, ZHAO Y J, LIAN F. Characteristics and electrochemical performance of cathode material Co-coated LiNiO2 for Li-ion batteries [J]. Trans Nonferrous Met Soc China, 2006, 16(1): 137-141.
[6] CEDER G, MISHRA S K. The stability of orthorhombic and monoclinic-layered LiMnO2 [J]. Electrochem. Solid-State Lett, 1999, 2(11): 550-552.
[7] YU L Y, QIU W H, LIAN F, LIU W, KANG X L, HUANG J Y. Comparative study of layered 0.65 Li[Li1/3Mn2/3]O2·0.35LiMO2 (M=Co, Ni1/2Mn1/2 and Ni1/3Co1/3Mn1/3) cathode materials [J]. Materials Letters, 2008, 62 (17/1): 3010-3013.
[8] LI Z G, WANG T D, KANG X Y. Synthesis and characterization of LiCo1/3Ni1/3Mn1/3O2 prepared by Pechini process [J]. Rare Metals, 2006, 27 (6): 7-11.
[9] SMART M C, WHITACRE J F, RATNAKUMAR B V, AMINE K. Electrochemical performance and kinetics of Li1+x(Co1/3Ni1/3Mn1/3)1-xO2 cathodes and graphite anodes in low-temperature electrolytes [J]. J Power Sources, 2007, 168(2): 501-508.
[10] SURENDRA K M, HADAR S, ZVI S F, DANIELA K. A comparative study of electrodes comprising nanometric and submicron particles of LiNi0.50Mn0.50O2, LiNi0.33Mn0.33Co0.33O2, and LiNi0.40Mn0.40Co0.20O2 layered compounds [J]. J Power Sources, 2009, 189(1): 248-255.
[11] HUI C, YAO Z, JIAN Z, BAO J X. Synthesis and electrochemical characteristics of layered LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries [J]. Solid State Ionics, 2005, 176(13/14): 1207-1211.
[12] NI J F, ZHOU H H, CHEN J T, ZHANG X X. Improved electrochemical performance of layered LiNi0.4Co0.2Mn0.4O2 via Li2ZrO3 coating [J]. Electrochimica Acta, 2008, 53(7): 3075-3083.
[13] KOSOVA N V, DEVYATKINA E T, KAICHEV V V. Optimization of Ni2+/Ni3+ ratio in layered Li(Ni,Mn,Co)O2 cathodes for better electrochemistry [J]. J Power Sources, 2007, 174: 965-969.
[14] LIU Z L, YU A S, LEE J Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries[J]. J Power Sources, 1999, 81/82: 416-419.
[15] LI J G, LI W, QIAN Z, HE X M. Synthesis and characterization of LiNi0.6Mn0.4-xCoxO2 as cathode materials for Li-ion batteries [J]. J Power Sources, 2009, 189(1): 28-33.
Foundation item: Projects(0991025, 0842003-5 and 0832259) supported by Natural Science Foundation of Guangxi Province, China; Project supported by the Joint Graduate Innovation Talent Cultivation Base of Guangxi Province, China; Project(GuiJiaoRen [2007]71) supported by the Research Funds of the Guangxi Key Laboratory of Environmental Engineering, Protection and Assessment Program to Sponsor Teams for Innovation in the Construction of Talent Highlands in Guangxi Institutions of Higher Learning, China
Corresponding author: ZHONG Sheng-kui; Tel: +86-773-5896446; E-mail: zhongshk@glite.edu.cn
DOI: 10.1016/S1003-6326(09)60059-5
(Edited by YANG Bing)